Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14
- PMID: 14517279
- PMCID: PMC2194215
- DOI: 10.1084/jem.20031076
Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14
Abstract
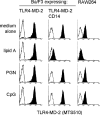
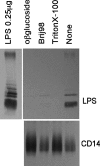
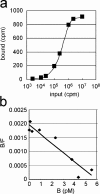
Toll-like receptors (TLRs) are innate recognition molecules for microbial products, but their direct interactions with corresponding ligands remain unclarified. LPS, a membrane constituent of gram-negative bacteria, is the best-studied TLR ligand and is recognized by TLR4 and MD-2, a molecule associated with the extracellular domain of TLR4. Although TLR4-MD-2 recognizes LPS, little is known about the physical interaction between LPS and TLR4-MD-2. Here, we demonstrate cell surface LPS-TLR4-MD-2 complexes. CD14 greatly enhances the formation of LPS-TLR4-MD-2 complexes, but is not coprecipitated with LPS-TLR4-MD-2 complexes, suggesting a role for CD14 in LPS loading onto TLR4-MD-2 but not in the interaction itself between LPS and TLR4-MD-2. A tentative dissociation constant (Kd) for LPS-TLR4-MD-2 complexes was approximately 3 nM, which is approximately 10-20 times lower than the reported Kd for LPS-MD-2 or LPS-CD14. The presence of detergent disrupts LPS interaction with CD14 but not with TLR4-MD-2. E5531, a lipid A antagonist developed for therapeutic intervention of endotoxin shock, blocks LPS interaction with TLR4-MD-2 at a concentration 100 times lower than that required for blocking LPS interaction with CD14. These results reveal direct LPS interaction with cell surface TLR4-MD-2 that is distinct from that with MD-2 or CD14.
Figures





References
-
- Janeway, C.A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. - PubMed
-
- Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376. - PubMed
-
- Ulevitch, R.J., and P.S. Tobias. 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13:437–457. - PubMed
-
- Wright, S.D., R.A. Ramos, P.S. Tobias, R.J. Ulevitch, and J.C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 249:1431–1433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

