Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis
- PMID: 14517293
- PMCID: PMC230332
- DOI: 10.1128/MCB.23.20.7230-7242.2003
Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis
Abstract
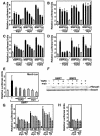
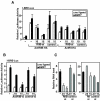
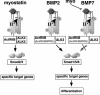
Myostatin, a transforming growth factor beta (TGF-beta) family member, is a potent negative regulator of skeletal muscle growth. In this study we characterized the myostatin signal transduction pathway and examined its effect on bone morphogenetic protein (BMP)-induced adipogenesis. While both BMP7 and BMP2 activated transcription from the BMP-responsive I-BRE-Lux reporter and induced adipogenic differentiation, myostatin inhibited BMP7- but not BMP2-mediated responses. To dissect the molecular mechanism of this antagonism, we characterized the myostatin signal transduction pathway. We showed that myostatin binds the type II Ser/Thr kinase receptor. ActRIIB, and then partners with a type I receptor, either activin receptor-like kinase 4 (ALK4 or ActRIB) or ALK5 (TbetaRI), to induce phosphorylation of Smad2/Smad3 and activate a TGF-beta-like signaling pathway. We demonstrated that myostatin prevents BMP7 but not BMP2 binding to its receptors and that BMP7-induced heteromeric receptor complex formation is blocked by competition for the common type II receptor, ActRIIB. Thus, our results reveal a strikingly specific antagonism of BMP7-mediated processes by myostatin and suggest that myostatin is an important regulator of adipogenesis.
Figures




References
-
- Aherns, M., T. Ankenbauer, D. Schroder, A. Hollnagel, H. Mayer, and G. Gross. 1993. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H 10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 12:871-880. - PubMed
-
- Asahina, I., T. K. Sampath, and P. V. Hauschka. 1996. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp. Cell Res. 222:38-47. - PubMed
-
- Attisano, L., J. Cárcamo, F. Ventura, F. M. B. Weis, J. Massagué, and J. L. Wrana. 1993. Identification of human activin and TGF-β type I receptors that form heteromeric kinase complexes with type II receptors. Cell 75:671-680. - PubMed
-
- Attisano, L., and J. L. Wrana. 2002. Signal transduction by the TGF-β superfamily. Science 296:1646-1647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
