Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates beta-catenin-mediated transcription
- PMID: 14517306
- PMCID: PMC230329
- DOI: 10.1128/MCB.23.20.7391-7402.2003
Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates beta-catenin-mediated transcription
Abstract
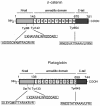
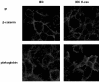
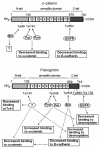
Plakoglobin is a protein closely related to beta-catenin that links desmosomal cadherins to intermediate filaments. Plakoglobin can also substitute for beta-catenin in adherens junctions, providing a connection between E-cadherin and alpha-catenin. Association of beta-catenin with E-cadherin and alpha-catenin is regulated by phosphorylation of specific tyrosine residues; modification of beta-catenin Tyr654 and Tyr142 decreases binding to E-cadherin and alpha-catenin, respectively. We show here that plakoglobin can also be phosphorylated on tyrosine residues, but unlike beta-catenin, this modification is not always associated with disrupted association with junctional components. Protein tyrosine kinases present distinct specificities on beta-catenin and plakoglobin, and phosphorylation of beta-catenin-equivalent Tyr residues of plakoglobin affects its interaction with components of desmosomes or adherens junctions differently. For instance, Src, which mainly phosphorylates Tyr86 in beta-catenin, modifies Tyr643 in plakoglobin, decreasing the interaction with E-cadherin and alpha-catenin and increasing the interaction with the alpha-catenin-equivalent protein in desmosomes, desmoplakin. The tyrosine kinase Fer, which modifies beta-catenin Tyr142, lessening its association with alpha-catenin, phosphorylates plakoglobin Tyr549 and exerts the contrary effect: it raises the binding of plakoglobin to alpha-catenin. These results suggest that tyrosine kinases like Src or Fer modulate desmosomes and adherens junctions differently. Our results also indicate that phosphorylation of Tyr549 and the increased binding of plakoglobin to components of adherens junctions can contribute to the upregulation of the transcriptional activity of the beta-catenin-Tcf-4 complex observed in many epithelial tumor cells.
Figures








Similar articles
-
Tyrosine phosphorylation of human keratinocyte beta-catenin and plakoglobin reversibly regulates their binding to E-cadherin and alpha-catenin.J Invest Dermatol. 2001 Nov;117(5):1059-67. doi: 10.1046/j.0022-202x.2001.01523.x. J Invest Dermatol. 2001. PMID: 11710913
-
Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells.J Cell Sci. 1997 Sep;110 ( Pt 17):2065-77. doi: 10.1242/jcs.110.17.2065. J Cell Sci. 1997. PMID: 9378757
-
Cross-talk between adherens junctions and desmosomes depends on plakoglobin.J Cell Biol. 1997 Feb 24;136(4):919-34. doi: 10.1083/jcb.136.4.919. J Cell Biol. 1997. PMID: 9049256 Free PMC article.
-
Cadherin-catenin complex: protein interactions and their implications for cadherin function.J Cell Biochem. 1996 Jun 15;61(4):514-23. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. J Cell Biochem. 1996. PMID: 8806074 Review.
-
Tyrosine phosphorylation and cadherin/catenin function.Bioessays. 1997 Oct;19(10):883-91. doi: 10.1002/bies.950191008. Bioessays. 1997. PMID: 9363682 Review.
Cited by
-
The role of Eph receptors in lens function and disease.Sci China Life Sci. 2012 May;55(5):434-43. doi: 10.1007/s11427-012-4318-7. Epub 2012 May 27. Sci China Life Sci. 2012. PMID: 22645087 Free PMC article. Review.
-
FER overexpression is associated with poor postoperative prognosis and cancer-cell survival in non-small cell lung cancer.Int J Clin Exp Pathol. 2013;6(4):598-612. Epub 2013 Mar 15. Int J Clin Exp Pathol. 2013. PMID: 23573306 Free PMC article.
-
A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair.J Cell Biol. 2012 Apr 30;197(3):381-9. doi: 10.1083/jcb.201107078. Epub 2012 Apr 23. J Cell Biol. 2012. PMID: 22529101 Free PMC article.
-
Mechanistic basis of desmosome-targeted diseases.J Mol Biol. 2013 Nov 1;425(21):4006-22. doi: 10.1016/j.jmb.2013.07.035. Epub 2013 Aug 2. J Mol Biol. 2013. PMID: 23911551 Free PMC article. Review.
-
Desmosomes: new perpetrators in tumour suppression.Nat Rev Cancer. 2011 May;11(5):317-23. doi: 10.1038/nrc3051. Nat Rev Cancer. 2011. PMID: 21508970 Free PMC article. Review.
References
-
- Aberle, H., H. Schwartz, H. Hoschuetzky, and R. Kemler. 1996. Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to α-catenin. J. Biol. Chem. 271:1520-1526. - PubMed
-
- Adams, C., and J. W. Nelson. 1998. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 10:572-577. - PubMed
-
- Behrens, J., L. Vakaet, R. Friis, E. Winterhager, F. Van Roy, M. Mareel, and W. Birchmeier. 1993. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120:757-766. - PMC - PubMed
-
- Bienz, M., and H. Clevers. 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311-320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
