The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast
- PMID: 14517318
- PMCID: PMC206999
- DOI: 10.1091/mbc.e03-04-0247
The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast
Abstract
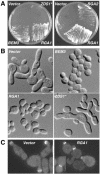
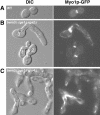
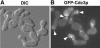
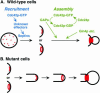
The septins are a conserved family of GTP-binding, filament-forming proteins. In the yeast Saccharomyces cerevisiae, the septins form a ring at the mother-bud neck that appears to function primarily by serving as a scaffold for the recruitment of other proteins to the neck, where they participate in cytokinesis and a variety of other processes. Formation of the septin ring depends on the Rho-type GTPase Cdc42p but appears to be independent of the actin cytoskeleton. In this study, we investigated further the mechanisms of septin-ring formation. Fluorescence-recovery-after-photobleaching (FRAP) experiments indicated that the initial septin structure at the presumptive bud site is labile (exchanges subunits freely) but that it is converted into a stable ring as the bud emerges. Mutants carrying the cdc42V36G allele or lacking two or all three of the known Cdc42p GTPase-activating proteins (GAPs: Bem3p, Rga1p, and Rga2p) could recruit the septins to the cell cortex but were blocked or delayed in forming a normal septin ring and had accompanying morphogenetic defects. These phenotypes were dramatically enhanced in mutants that were also defective in Cla4p or Gin4p, two protein kinases previously shown to be important for normal septin-ring formation. The Cdc42p GAPs colocalized with the septins both early and late in the cell cycle, and overexpression of the GAPs could suppress the septin-organization and morphogenetic defects of temperature-sensitive septin mutants. Taken together, the data suggest that formation of the mature septin ring is a process that consists of at least two distinguishable steps, recruitment of the septin proteins to the presumptive bud site and their assembly into the stable septin ring. Both steps appear to depend on Cdc42p, whereas the Cdc42p GAPs and the other proteins known to promote normal septin-ring formation appear to function in a partially redundant manner in the assembly step. In addition, because the eventual formation of a normal septin ring in a cdc42V36G or GAP mutant was invariably accompanied by a switch from an abnormally elongated to a more normal bud morphology distal to the ring, it appears that the septin ring plays a direct role in determining the pattern of bud growth.
Figures






References
-
- Ayscough, K.R., Stryker, J., Pokala, N., Sanders, M., Crews, P., and Drubin, D.G. (1997). High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor Latrunculin-A. J. Cell Biol. 137, 399–416. - PMC - PubMed
-
- Barral, Y., Mermall, V., Mooseker, M.S., and Snyder, M. (2000). Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5, 841–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

