Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion
- PMID: 14517330
- PMCID: PMC207012
- DOI: 10.1091/mbc.e03-03-0147
Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion
Abstract
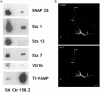
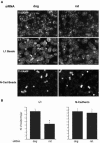
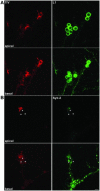
The membrane-trafficking pathway mediated by tetanus neurotoxin-insensitive vesicle-associated membrane protein (TI-VAMP) in neurons is still unknown. We show herein that TI-VAMP expression is necessary for neurite outgrowth in PC12 cells and hippocampal neurons in culture. TI-VAMP interacts with plasma membrane and endosomal target soluble N-ethylmaleimide-sensitive factor attachment protein receptors, suggesting that TI-VAMP mediates a recycling pathway. L1, a cell-cell adhesion molecule involved in axonal outgrowth, colocalized with TI-VAMP in the developing brain, neurons in culture, and PC12 cells. Plasma membrane L1 was internalized into the TI-VAMP-containing compartment. Silencing of TI-VAMP resulted in reduced expression of L1 at the plasma membrane. Finally, using the extracellular domain of L1 and N-cadherin immobilized on beads, we found that the silencing of TI-VAMP led to impaired L1- but not N-cadherin-mediated adhesion. Furthermore, TI-VAMP- but not synaptobrevin 2-containing vesicles accumulated at the site of the L1 bead-cell junction. We conclude that TI-VAMP mediates the intracellular transport of L1 and that L1-mediated adhesion controls this membrane trafficking, thereby suggesting an important cross talk between membrane trafficking and cell-cell adhesion.
Figures






References
-
- Berditchevski, F. (2001). Complexes of tetraspanins with integrins: more than meets the eye. J. Cell Sci. 114, 4143–4151. - PubMed
-
- Borgonovo, B., Cocucci, E., Racchetti, G., Podini, P., Bachi, A., and Meldolesi, J. (2002). Regulated exocytosis: a novel, widely expressed system. Nat. Cell Biol. 4, 955–962. - PubMed
-
- Brummendorf, T., Kenwrick, S., and Rathjen, F.G. (1998). Neural cell recognition molecule L 1, from cell biology to human hereditary brain malformations. Curr. Opin. Neurobiol. 8, 87–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

