Mitogen-activated protein kinase stimulation of Ca(2+) signaling is required for survival of endoplasmic reticulum stress in yeast
- PMID: 14517337
- PMCID: PMC207020
- DOI: 10.1091/mbc.e03-02-0113
Mitogen-activated protein kinase stimulation of Ca(2+) signaling is required for survival of endoplasmic reticulum stress in yeast
Abstract
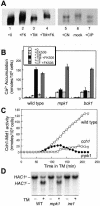
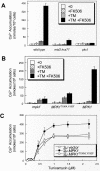
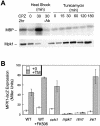
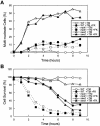
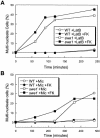
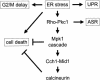
Endoplasmic reticulum (ER) stress in the budding yeast Saccharomyces cerevisiae triggers Ca2+ influx through a plasma membrane channel composed of Cch1 and Mid1. This response activates calcineurin, which helps to prevent cell death during multiple forms of ER stress, including the response to azole-class antifungal drugs. Herein, we show that ER stress activates the cell integrity mitogen-activate protein kinase cascade in yeast and that the activation of Pkc1 and Mpk1 is necessary for stimulation of the Cch1-Mid1 Ca2+ channel independent of many known targets of Mpk1 (Rlm1, Swi4, Swi6, Mih1, Hsl1, and Swe1). ER stress generated in response to miconazole, tunicamycin, or other inhibitors also triggered a transient G2/M arrest that depended upon the Swe1 protein kinase. Calcineurin played little role in the Swe1-dependent cell cycle arrest and Swe1 had little effect on calcineurin-dependent avoidance of cell death. These findings help to clarify the interactions between Mpk1, calcineurin, and Swe1 and suggest that the calcium cell survival pathway promotes drug resistance independent of both the unfolded protein response and the G2/M cell cycle checkpoint.
Figures

References
-
- Cruz, M.C., Del Poeta, M., Wang, P., Wenger, R., Zenke, G., Quesniaux, V.F., Movva, N.R., Perfect, J.R., Cardenas, M.E., and Heitman, J. (2000). Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob. Agents Chemother. 44, 143–149. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

