Four-dimensional multiphoton imaging of brain entry, amyloid binding, and clearance of an amyloid-beta ligand in transgenic mice
- PMID: 14517353
- PMCID: PMC218780
- DOI: 10.1073/pnas.2034101100
Four-dimensional multiphoton imaging of brain entry, amyloid binding, and clearance of an amyloid-beta ligand in transgenic mice
Abstract
The lack of a specific biomarker makes preclinical diagnosis of Alzheimer's disease (AD) impossible, and it precludes assessment of therapies aimed at preventing or reversing the course of the disease. The development of a tool that enables direct, quantitative detection of the amyloid-beta deposits found in the disease would provide an excellent biomarker. This article demonstrates the real-time biodistribution kinetics of an imaging agent in transgenic mouse models of AD. Using multiphoton microscopy, Pittsburgh compound B (PIB) was imaged with sub-microm resolution in the brains of living transgenic mice during peripheral administration. PIB entered the brain quickly and labeled amyloid deposits within minutes. The nonspecific binding was cleared rapidly, whereas specific labeling was prolonged. WT mice showed rapid brain entry and clearance of PIB without any binding. These results demonstrate that the compound PIB has the properties required for a good amyloid-imaging agent in humans with or at risk for AD.
Figures



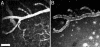
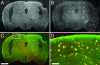
References
-
- Hyman, B. T. & Trojanowski, J. Q. (1997) J. Neuropathol. Exp. Neurol. 56, 1095-1097. - PubMed
-
- Markesbery, W. R. (1997) Neurobiol. Aging 18, S13-S19. - PubMed
-
- Klunk, W. E. (1998) Neurobiol. Aging 19, 145-147. - PubMed
-
- Eckelman, W. C. (2002) Nucl. Med. Biol. 29, 777-782. - PubMed
-
- Shoghi-Jadid, K., Small, G. W., Agdeppa, E. D., Kepe, V., Ercoli, L. M., Siddarth, P., Read, S., Satyamurthy, N., Petric, A., Huang, S. C. & Barrio, J. R. (2002) Am. J. Geriatr. Psychiatry 10, 24-35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG020570/AG/NIA NIH HHS/United States
- AG 020570/AG/NIA NIH HHS/United States
- R01 AG018402/AG/NIA NIH HHS/United States
- AG 08487/AG/NIA NIH HHS/United States
- R01 AG008487/AG/NIA NIH HHS/United States
- P01 AG015453/AG/NIA NIH HHS/United States
- AG 15453/AG/NIA NIH HHS/United States
- R01 EB000768/EB/NIBIB NIH HHS/United States
- EB 00768/EB/NIBIB NIH HHS/United States
- R01 AG020226/AG/NIA NIH HHS/United States
- AG 20226/AG/NIA NIH HHS/United States
- K02 AG001039/AG/NIA NIH HHS/United States
- AG 18402/AG/NIA NIH HHS/United States
- AG 01039/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

