KRAB-containing zinc-finger repressor proteins
- PMID: 14519192
- PMCID: PMC328446
- DOI: 10.1186/gb-2003-4-10-231
KRAB-containing zinc-finger repressor proteins
Abstract
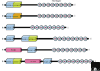
The largest family of zinc-finger transcription factors comprises those containing the Krüppel-associated box (or KRAB domain), which are present only in tetrapod vertebrates. Many genes encoding KRAB-containing proteins are arranged in clusters in the human genome, with one cluster close to chromosome 9ql3 and others in centromeric and telomeric regions of other chromosomes, but other genes occur individually throughout the genome. The KRAB domain, which is found in the amino-terminal region of the proteins, behaves as a transcriptional repressor domain by binding to corepressor proteins, whereas the C2H2 zinc-finger motifs bind DNA. The functions currently proposed for members of the KRAB-containing protein family include transcriptional repression of RNA polymerase I, II, and III promoters and binding and splicing of RNA. Members of the family are involved in maintenance of the nucleolus, cell differentiation, cell proliferation, apoptosis, and neoplastic transformation.
Figures

References
-
- Bellefroid EJ, Poncelet DA, Lecocq PJ, Revelant O, Martial JA. The evolutionarily conserved Kruppel-associated box domain defines a subfamily of eukaryotic multifingered proteins. Proc Natl Acad Sci USA. 1991;88:3608–3612. The authors show that the KRAB domain is present in about one-third of zinc-finger proteins analyzed and that it is a conserved domain of 75 amino acids located in the amino-terminal portion of these proteins. - PMC - PubMed
-
- Rousseau-Merck MF, Koczan D, Legrand I, Moller S, Autran S, Thiesen HJ. The KOX zinc finger genes: genome wide mapping of 368 ZNF PAC clones with zinc finger gene clusters predominantly in 23 chromosomal loci are confirmed by human sequences annotated in EnsEMBL. Cytogenet Genome Res. 2002;98:147–153. doi: 10.1159/000069802. In this article, the authors generated phylogenetic trees of all KRAB-containing human zinc-finger proteins with the goal of documenting their evolution in primates. - DOI - PubMed
-
- Looman C, Abrink M, Mark C, Hellman L. KRAB zinc finger proteins: an analysis of the molecular mechanisms governing their increase in numbers and complexity during evolution. Mol Biol Evol. 2002;19:2118–2130. This article shows that both the KRAB A + b and the KRAB A subfamilies of zinc-finger proteins may have originated from a single member or a few closely related members of the KRAB A + B family. The KRAB A + B family is also the most prevalent among the KRAB zinc-finger genes. - PubMed
-
- Witzgall R, O'Leary E, Leaf A, Onaldi D, Bonventre JV. The Kruppel-associated box-A (KRAB-A) domain of zinc finger proteins mediates transcriptional repression. Proc Natl Acad Sci USA. 1994;91:4514–4518. KRAB domains can inhibit the activating function of known transcriptional regulators, and the KRAB domain silences both activated and basal promoter activity of TATA-containing promoters. - PMC - PubMed
-
- Gebelein B, Urrutia R. Sequence-specific transcriptional repression by KS1, a multiple-zinc- finger-Kruppel-associated box protein. Mol Cell Biol. 2001;21:928–939. doi: 10.1128/MCB.21.3.928-939.2001. This article demonstrates that KRAB-containing proteins have a sequence-specific repression function and characterizes the manner by which these proteins bind to DNA. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

