Automated laboratory reporting of infectious diseases in a climate of bioterrorism
- PMID: 14519239
- PMCID: PMC3016787
- DOI: 10.3201/eid0909.020486
Automated laboratory reporting of infectious diseases in a climate of bioterrorism
Abstract
While newly available electronic transmission methods can increase timeliness and completeness of infectious disease reports, limitations of this technology may unintentionally compromise detection of, and response to, bioterrorism and other outbreaks. We reviewed implementation experiences for five electronic laboratory systems and identified problems with data transmission, sensitivity, specificity, and user interpretation. The results suggest a need for backup transmission methods, validation, standards, preserving human judgment in the process, and provider and end-user involvement. As illustrated, challenges encountered in deployment of existing electronic laboratory reporting systems could guide further refinement and advances in infectious disease surveillance.
Figures
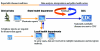
References
-
- Centers for Disease Control and Prevention. An overview of NEDSS initiative. [Cited June 12, 2002.] Available from: URL: http://www.cdc.gov/nedss/About/overview.html.
-
- U.S. Department of Health and Human Services. FY2002. Washington, DC: The Department; 2002. [Cited June 4, 2002.] Available from: URL: http://www.hhs.gov/ophp/funding.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
