Mammary epithelial stem cells: transplantation and self-renewal analysis
- PMID: 14521512
- PMCID: PMC6495449
- DOI: 10.1046/j.1365-2184.36.s.1.2.x
Mammary epithelial stem cells: transplantation and self-renewal analysis
Abstract
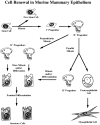
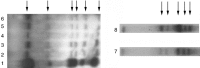
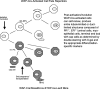
An entire mammary epithelial outgrowth, capable of full secretory differentiation, may be comprised of the progeny of a single cellular antecedent. This conclusion is based upon the maintenance of retroviral insertion sites within the somatic DNA of successive transplant generations derived from a single mammary fragment. In addition, dissociation of these clonal dominant glands and implantation of dispersed cells at limiting dilution demonstrated that both duct-limited and lobule-limited outgrowths were developed, as well as complete, fully differentiated glands. Thus, transplantation has revealed three distinct mammary epithelial progenitors in the mouse. Similar studies have extended this observation to rat mammary tissue. Recently, using cre-lox conditional activation of reporter genes, a new epithelial progenitor, specific for mammary secretory epithelium in postlactation females has been uncovered. In situ, these cells were shown to regenerate secretory lobules upon successive pregnancies. In transplant studies, they demonstrated the capacity for self-renewal and contributed to the new generation of all of the known epithelial cell types among mammary epithelium. In limiting dilution, the parity-induced progenitors were capable of engendering lobule-limited and duct-limited outgrowths in their entirety, but not completely developed glands. Serial transplant studies indicate that these progenitors have a significant but limited capacity for self-renewal.
Figures

Similar articles
-
Stem cells and the mammary microenvironment.Breast Dis. 2008;29:57-67. doi: 10.3233/bd-2008-29107. Breast Dis. 2008. PMID: 19029625 Free PMC article.
-
Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression.Oncogene. 2005 Jan 20;24(4):552-60. doi: 10.1038/sj.onc.1208185. Oncogene. 2005. PMID: 15580303
-
An entire functional mammary gland may comprise the progeny from a single cell.Development. 1998 May;125(10):1921-30. doi: 10.1242/dev.125.10.1921. Development. 1998. PMID: 9550724
-
Mammary epithelial stem cells.Microsc Res Tech. 2001 Jan 15;52(2):190-203. doi: 10.1002/1097-0029(20010115)52:2<190::AID-JEMT1005>3.0.CO;2-O. Microsc Res Tech. 2001. PMID: 11169867 Review.
-
Murine mammary epithelial stem cells: discovery, function, and current status.Cold Spring Harb Perspect Biol. 2011 Feb 1;3(2):a004879. doi: 10.1101/cshperspect.a004879. Cold Spring Harb Perspect Biol. 2011. PMID: 20926515 Free PMC article. Review.
Cited by
-
Stem cells and tissue homeostasis in mammary glands.J Mammary Gland Biol Neoplasia. 2005 Jan;10(1):1-3. doi: 10.1007/s10911-005-2535-4. J Mammary Gland Biol Neoplasia. 2005. PMID: 15886881 No abstract available.
-
Contribution of an alveolar cell of origin to the high-grade malignant phenotype of pregnancy-associated breast cancer.Oncogene. 2014 Dec 11;33(50):5729-39. doi: 10.1038/onc.2013.521. Epub 2013 Dec 9. Oncogene. 2014. PMID: 24317513 Free PMC article.
-
The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium.Breast Cancer Res. 2006;8(2):207. doi: 10.1186/bcr1411. Epub 2006 Apr 25. Breast Cancer Res. 2006. PMID: 16677418 Free PMC article. Review.
-
Expression of Notch receptors, ligands, and target genes during development of the mouse mammary gland.J Cell Physiol. 2011 Jul;226(7):1940-52. doi: 10.1002/jcp.22526. J Cell Physiol. 2011. PMID: 21506125 Free PMC article.
-
Wnt signaling, stem cells, and the cellular origin of breast cancer.Stem Cell Rev. 2007 Jun;3(2):157-68. doi: 10.1007/s12015-007-0025-3. Stem Cell Rev. 2007. PMID: 17873348 Review.
References
-
- Boulanger CA, Smith GH (2001) Reducing mammary cancer risk through premature stem cell senescence. Oncogene 20, 2264. - PubMed
-
- Buggiano V, Schere‐Levy C, Abe K, Vanzulli S, Piazzon I, Smith GH, Kordon EC (2001) Impairment of mammary lobular development induced by expression of TGFβ1 under the control of the WAP promoter does not suppress tumorigenesis in MMTV‐infected transgenic mice. Int. J. Cancer 92, 568. - PubMed
-
- Chepko G, Smith GH (1997) Three division‐competent, structurally‐distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell. 29, 239. - PubMed
-
- Daniel CW, Aidells BD, Medina D, Faulkin LJ (1975) Unlimited division potential of precancerous mouse mammary cells after spontaneous or carcinogen‐induced transformation. Fed. Proc. 34, 64. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

