Heart valve and arterial tissue engineering
- PMID: 14521518
- PMCID: PMC6496809
- DOI: 10.1046/j.1365-2184.2003.00281.x
Heart valve and arterial tissue engineering
Abstract
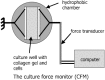
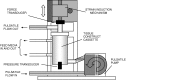
In the industrialized world, cardiovascular disease alone is responsible for almost half of all deaths. Many of the conditions can be treated successfully with surgery, often using transplantation techniques; however, autologous vessels or human-donated organs are in short supply. Tissue engineering aims to create specific, matching grafts by growing cells on appropriate matrices, but there are many steps between the research laboratory and the operating theatre. Neo-tissues must be effective, durable, non-thrombogenic and non-immunogenic. Scaffolds should be bio-compatible, porous (to allow cell/cell communication) and amenable to surgery. In the early days of cardiovascular tissue engineering, autologous or allogenic cells were grown on inert matrices, but patency and thrombogenicity of grafts were disappointing. The current ethos is toward appropriate cell types grown in (most often) a polymeric matrix that degrades at a rate compatible with the cells' production of their own extracellular matrical proteins, thus gradually replacing the graft with a living counterpart. The geometry is crucial. Computer models have been made of valves, and these are used as three-dimensional patterns for mass-production of implant scaffolds. Vessel walls have integral connective tissue architecture, and application of physiological level mechanical forces conditions bio-engineered components to align in precise orientation. This article reviews the concepts involved and successes achieved to date.
Figures




Similar articles
-
Tissue engineering of autologous human heart valves using cryopreserved vascular umbilical cord cells.Ann Thorac Surg. 2006 Jun;81(6):2207-16. doi: 10.1016/j.athoracsur.2005.12.073. Ann Thorac Surg. 2006. PMID: 16731156
-
[Heart valve and myocardial tissue engineering].Herz. 2010 Aug;35(5):334-41. doi: 10.1007/s00059-010-3355-x. Herz. 2010. PMID: 20631970 Review. German.
-
Engineering of arteries in vitro.Cell Mol Life Sci. 2014 Jun;71(11):2103-18. doi: 10.1007/s00018-013-1546-3. Epub 2014 Jan 8. Cell Mol Life Sci. 2014. PMID: 24399290 Free PMC article. Review.
-
Tissue engineering on matrix: future of autologous tissue replacement.Semin Immunopathol. 2011 May;33(3):307-15. doi: 10.1007/s00281-011-0258-8. Epub 2011 Jan 29. Semin Immunopathol. 2011. PMID: 21279358 Review.
-
Rapid manufacturing techniques for the tissue engineering of human heart valves.Eur J Cardiothorac Surg. 2014 Oct;46(4):593-601. doi: 10.1093/ejcts/ezt510. Epub 2014 Jul 25. Eur J Cardiothorac Surg. 2014. PMID: 25063052 Review.
Cited by
-
Polymer scaffolds for small-diameter vascular tissue engineering.Adv Funct Mater. 2010 Sep 9;20(17):2833-2841. doi: 10.1002/adfm.201000922. Adv Funct Mater. 2010. PMID: 24501590 Free PMC article.
-
Learning-enhanced 3D fiber orientation mapping in thick cardiac tissues.Biomed Opt Express. 2025 Jul 22;16(8):3315-3336. doi: 10.1364/BOE.563643. eCollection 2025 Aug 1. Biomed Opt Express. 2025. PMID: 40809973 Free PMC article.
References
-
- Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA (2000) Hepatocytes from non‐hepatic adult stem cells. Nature 406, 257. - PubMed
-
- Barker JN, Wagner JE (2002) Umbilical cord blood transplantation: current state of the art. Curr. Opin. Oncol. 14, 160. - PubMed
-
- Becker NA, Kelm RJ, Vrana JA, Getz MJ, Maher LJ (2000) Altered sensitivity to single‐strand‐specific reagents associated with the genomic vascular smooth muscle alpha‐actin promoter during myofibroblast differentiation. J. Bio. Chem. 275, 15384. - PubMed
-
- Bots JGF, Van Der Does L, Bantjes A (1986) Small diameter blood vessel prostheses from blends of polyethylene oxide and polypropaline oxide. Biomaterials 7, 393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

