A common polymorphism in the oxygen-dependent degradation (ODD) domain of hypoxia inducible factor-1alpha (HIF-1alpha) does not impair Pro-564 hydroxylation
- PMID: 14521712
- PMCID: PMC212228
- DOI: 10.1186/1476-4598-2-31
A common polymorphism in the oxygen-dependent degradation (ODD) domain of hypoxia inducible factor-1alpha (HIF-1alpha) does not impair Pro-564 hydroxylation
Abstract
Background: The hypoxia-inducible factor (HIF) transcription complex, which is activated by low oxygen tension, controls a diverse range of cellular processes including angiogenesis and erythropoiesis. Under normoxic conditions, the alpha subunit of HIF is rapidly degraded in a manner dependent on hydroxylation of two conserved proline residues at positions 402 and 564 in HIF-1alpha in the oxygen-dependent degradation (ODD) domain. This allows subsequent recognition by the von Hippel-Lindau (VHL) tumor suppressor protein, which targets HIF for degradation by the ubiquitin-proteasome pathway. Under hypoxic conditions, prolyl hydroxylation of HIF is inhibited, allowing it to escape VHL-mediated degradation. The transcriptional regulation of the erythropoietin gene by HIF raises the possibility that HIF may play a role in disorders of erythropoiesis, such as idiopathic erythrocytosis (IE).
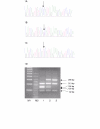
Results: Patients with IE were screened for changes in the HIF-1alpha coding sequence, and a change in the ODD domain that converts Pro-582 to Ser was identified in several patients. This same change, however, was also detected at a significant frequency, 0.073, in unaffected controls compared to 0.109 in the IE patient group. In vitro hydroxylation assays examining this amino acid change failed to reveal a discernible effect on HIF hydroxylation at Pro-564.
Conclusion: The Pro582Ser change represents a common polymorphism of HIF-1alpha that does not impair HIF-1alpha prolyl hydroxylation. Although the Pro582Ser polymorphism is located in the ODD domain of HIF-1alpha it does not diminish the association of HIF-1alpha with VHL. Thus, it is unlikely that this polymorphism accounts for the erythrocytosis in the group of IE patients studied.
Figures

References
-
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

