Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis
- PMID: 14522949
- PMCID: PMC218082
- DOI: 10.1101/gad.277003
Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis
Abstract
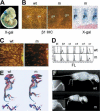
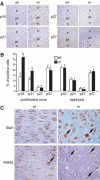
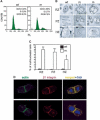
Beta1 integrins are highly expressed on chondrocytes, where they mediate adhesion to cartilage matrix proteins. To assess the functions of beta1 integrin during skeletogenesis, we inactivated the beta1 integrin gene in chondrocytes. We show here that these mutant mice develop a chondrodysplasia of various severity. beta1-deficient chondrocytes had an abnormal shape and failed to arrange into columns in the growth plate. This is caused by a lack of motility, which is in turn caused by a loss of adhesion to collagen type II, reduced binding to and impaired spreading on fibronectin, and an abnormal F-actin organization. In addition, mutant chondrocytes show decreased proliferation caused by a defect in G1/S transition and cytokinesis. The G1/S defect is, at least partially, caused by overexpression of Fgfr3, nuclear translocation of Stat1/Stat5a, and up-regulation of the cell cycle inhibitors p16 and p21. Altogether these findings establish that beta1-integrin-dependent motility and proliferation of chondrocytes are mandatory events for endochondral bone formation to occur.
Figures




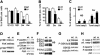
References
-
- Beier F., Leask, T.A., Haque, S., Chow, C., Taylor, A.C., Lee, R.J., Pestell, R.G., Ballock, R.T., and LuValle, P. 1999. Cell cycle genes in chondrocyte proliferation and differentiation. Matrix Biol. 18: 109-120. - PubMed
-
- Bellissent-Waydelich A., Vanier, M.T., Albiges-Rizo, C., and Simon-Assmann, P. 1999. Talin concentrates to the mid-body region during mammalian cell cytokinesis. J. Histochem. Cytochem. 47: 1357-1368. - PubMed
-
- Bouvard D., Brakebusch, C., Gustafsson, E., Aszodi, A., Bengtsson, T., Berna, A., and Fässler, R. 2001. Functional consequences of integrin gene mutations in mice. Circ. Res. 89: 211-223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
