Long-lasting activation of rhythmic neuronal activity by a novel mechanosensory system in the crustacean stomatogastric nervous system
- PMID: 14523066
- PMCID: PMC6494456
- DOI: 10.1152/jn.00741.2003
Long-lasting activation of rhythmic neuronal activity by a novel mechanosensory system in the crustacean stomatogastric nervous system
Abstract
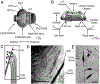
Sensory neurons enable neural circuits to generate behaviors appropriate for the current environmental situation. Here, we characterize the actions of a population (about 60) of bilaterally symmetric bipolar neurons identified within the inner wall of the cardiac gutter, a foregut structure in the crab Cancer borealis. These neurons, called the ventral cardiac neurons (VCNs), project their axons through the crab stomatogastric nervous system to influence neural circuits associated with feeding. Brief pressure application to the cardiac gutter transiently modulated the filtering motor pattern (pyloric rhythm) generated by the pyloric circuit within the stomatogastric ganglion (STG). This modulation included an increased speed of the pyloric rhythm and a concomitant decrease in the activity of the lateral pyloric neuron. Furthermore, 2 min of rhythmic pressure application to the cardiac gutter elicited a chewing motor pattern (gastric mill rhythm) generated by the gastric mill circuit in the STG that persisted for < or =30 min. These sensory actions on the pyloric and gastric mill circuits were mimicked by either ventral cardiac nerve or dorsal posterior esophageal nerve stimulation. VCN actions on the STG circuits required the activation of projection neurons in the commissural ganglia. A subset of the VCN actions on these projection neurons appeared to be direct and cholinergic. We propose that the VCN neurons are mechanoreceptors that are activated when food stored in the foregut applies an outward force, leading to the long-lasting activation of projection neurons required to initiate chewing and modify the filtering of chewed food.
Figures


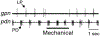

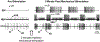

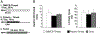




References
-
- Akay T, Bassler U, Gerharz P, and Buschges A. The role of sensory signals from the insect coxa-trochanta joint in controlling motor activity of the femur-tibia joint. J Neurophysiol 85: 594–604, 2001. - PubMed
-
- Alexandrowitcz JS. Muscle receptor organs in the abdomen of Homarus vulgaris and Palinurus vulgaris. Q J Microscop Sci 92: 163–203, 1951.
-
- Andersson O and Grillner S. Peripheral control of the cat’s step cycle. I. Phase dependent effects of ramp-movements of the hip during “fictive locomotion.” Acta Physiol Scand 113: 89–101, 1981. - PubMed
-
- Beenhakker MP, Hertzberg S, and Nusbaum MP. Neural network modulation by mechanosensory activation. Soc Neurosci Abstr 26: 449, 2000.
-
- Bennet-Clark HC. Abdominal stretch and inhibition of moulting in Rhodnius prolixus (hemiptera). J Insect Physiol 12: 1019–1028, 1966.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

