Proteasome inhibition stabilizes tau inclusions in oligodendroglial cells that occur after treatment with okadaic acid
- PMID: 14523089
- PMCID: PMC6740385
- DOI: 10.1523/JNEUROSCI.23-26-08872.2003
Proteasome inhibition stabilizes tau inclusions in oligodendroglial cells that occur after treatment with okadaic acid
Abstract
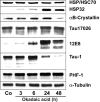
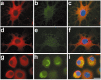
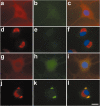
Tau-positive inclusions in oligodendrocytes are consistent neuropathological features of corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementias with Parkinsonism linked to chromosome 17. Here we show by immunohistochemistry that tau-positive oligodendroglial inclusion bodies also contain the small heat-shock protein (HSP) alphaB-crystallin but not HSP70. To study the molecular mechanisms underlying inclusion body formation, we engineered an oligodendroglia cell line (OLN-t40) to overexpress the longest human tau isoform. Treatment of OLN-t40 cells with okadaic acid (OA), an inhibitor of protein phosphatase 2A, caused tau hyperphosphorylation and a decrease in the binding of tau to microtubules. Simultaneously, tau-positive aggregates that also stained with the amyloid-binding dye thioflavin-S as well as with antibodies to tau and alphaB-crystallin were detected. However, they were only transiently expressed and were degraded within 24 hr. When the proteasomal apparatus was inhibited by carbobenzoxy-l-leucyl-l-leucyl-l-leucinal (MG-132) after OA treatment, the aggregates were stabilized and were still detectable after 18 hr in the absence of OA. Incubation with MG-132 alone inhibited tau proteolysis and led to the induction of HSPs, including alphaB-crystallin and to its translocation to the perinuclear region, but did not induce the formation of thioflavin-S-positive aggregates. Hence, although tau hyperphosphorylation induced by protein phosphatase inhibition contributes to pathological aggregate formation, only hyperphosporylation of tau followed by proteasome inhibition leads to stable fibrillary deposits of tau similar to those observed in neurodegenerative diseases.
Figures








Similar articles
-
Proteolytic stress causes heat shock protein induction, tau ubiquitination, and the recruitment of ubiquitin to tau-positive aggregates in oligodendrocytes in culture.J Neurosci. 2004 Jun 23;24(25):5748-57. doi: 10.1523/JNEUROSCI.1307-04.2004. J Neurosci. 2004. PMID: 15215297 Free PMC article.
-
Tau-inclusion body formation in oligodendroglia: the role of stress proteins and proteasome inhibition.Int J Dev Neurosci. 2004 Nov;22(7):443-51. doi: 10.1016/j.ijdevneu.2004.07.003. Int J Dev Neurosci. 2004. PMID: 15465274 Review.
-
alpha-Synuclein promotes the recruitment of tau to protein inclusions in oligodendroglial cells: effects of oxidative and proteolytic stress.J Mol Neurosci. 2009 Sep;39(1-2):226-34. doi: 10.1007/s12031-009-9190-y. Epub 2009 Mar 6. J Mol Neurosci. 2009. PMID: 19266322
-
Expression of the small heat-shock protein alphaB-crystallin in tauopathies with glial pathology.Am J Pathol. 2004 Jan;164(1):155-66. doi: 10.1016/s0002-9440(10)63106-9. Am J Pathol. 2004. PMID: 14695329 Free PMC article.
-
Protein aggregate formation in oligodendrocytes: tau and the cytoskeleton at the intersection of neuroprotection and neurodegeneration.Biol Chem. 2016 Mar;397(3):185-94. doi: 10.1515/hsz-2015-0157. Biol Chem. 2016. PMID: 26083267 Review.
Cited by
-
Glial cell inclusions and the pathogenesis of neurodegenerative diseases.Neuron Glia Biol. 2004 Feb;1(1):13-21. doi: 10.1017/s1740925x04000043. Neuron Glia Biol. 2004. PMID: 16614753 Free PMC article.
-
Oxidative stress promotes uptake, accumulation, and oligomerization of extracellular α-synuclein in oligodendrocytes.J Mol Neurosci. 2014 Mar;52(3):339-52. doi: 10.1007/s12031-013-0154-x. Epub 2013 Nov 12. J Mol Neurosci. 2014. PMID: 24217795
-
The cytoskeleton in oligodendrocytes. Microtubule dynamics in health and disease.J Mol Neurosci. 2008 May;35(1):55-63. doi: 10.1007/s12031-007-9017-7. Epub 2007 Dec 4. J Mol Neurosci. 2008. PMID: 18058074 Review.
-
Person-specific differences in ubiquitin-proteasome mediated proteostasis in human neurons.Alzheimers Dement. 2024 Apr;20(4):2952-2967. doi: 10.1002/alz.13680. Epub 2024 Mar 12. Alzheimers Dement. 2024. PMID: 38470006 Free PMC article.
-
The fine-tuning of proteolytic pathways in Alzheimer's disease.Cell Mol Life Sci. 2016 Sep;73(18):3433-51. doi: 10.1007/s00018-016-2238-6. Epub 2016 Apr 27. Cell Mol Life Sci. 2016. PMID: 27120560 Free PMC article. Review.
References
-
- Arai H, Atomi Y ( 1997) Chaperone activity of αB-crystallin suppresses tubulin aggregation through complex formation. Cell Struct Funct 22: 539-544. - PubMed
-
- Berry RW, Quinn B, Johnson N, Binder LI ( 2001) Pathological tau accumulations in neurodegenerative disease: review and case report. Neurochem Int 39: 469-479. - PubMed
-
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM-Y ( 1993) Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron 10: 1089-1099. - PubMed
-
- Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR ( 2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33: 95-130. - PubMed
-
- Cairns NJ, Atkinson PF, Hanger DP, Anderton BH, Daniel SE, Lantos P ( 1997) Tau protein in glial cytoplasmic inclusions of multiple system atrophy can be distinguished from abnormal tau in Alzheimer's disease. Neurosci Lett 230: 49-52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
