Thrombomodulin allosterically modulates the activity of the anticoagulant thrombin
- PMID: 14523228
- PMCID: PMC218711
- DOI: 10.1073/pnas.2135346100
Thrombomodulin allosterically modulates the activity of the anticoagulant thrombin
Abstract
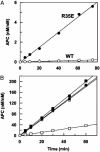
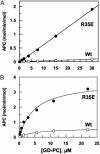
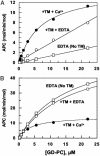
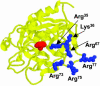
Exosite 1 of thrombin consists of a cluster of basic residues (Arg-35, Lys-36, Arg-67, Lys-70, Arg-73, Arg-75, and Arg-77 in chymotrypsinogen numbering) that play key roles in the function of thrombin. Structural data suggest that the side chain of Arg-35 projects toward the active site pocket of thrombin, but all other residues are poised to interact with thrombomodulin (TM). To study the role of these residues in TM-mediated protein C (PC) activation by thrombin, a charge reversal mutagenesis approach was used to replace these residues with a Glu in separate constructs. The catalytic properties of the mutants toward PC were analyzed in both the absence and presence of TM and Ca2+. It was discovered that, with the exception of the Arg-67 and Lys-70 mutants, all other mutants activated PC with similar maximum rate constants in the presence of a saturating concentration of TM and Ca2+, although their affinity for interaction with TM was markedly impaired. The catalytic properties of the Arg-35 mutant were changed so that PC activation by the mutant no longer required Ca2+ in the presence of TM, but, instead, it was accelerated by EDTA. Moreover, the activity of this mutant toward PC was improved approximately 25-fold independent of TM. These results suggest that Arg-35 is responsible for the Ca2+ dependence of PC activation by the thrombin-TM complex and that a function for TM in the activation complex is the allosteric alleviation of the inhibitory interaction of Arg-35 with the substrate.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

