Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2
- PMID: 14523231
- PMCID: PMC218749
- DOI: 10.1073/pnas.2133476100
Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2
Abstract
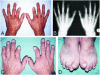
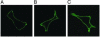
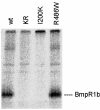
Brachydactyly (BD) type A2 is an autosomal dominant hand malformation characterized by shortening and lateral deviation of the index fingers and, to a variable degree, shortening and deviation of the first and second toes. We performed linkage analysis in two unrelated German families and mapped a locus for BD type A2 to 4q21-q25. This interval includes the gene bone morphogenetic protein receptor 1B (BMPR1B), a type I transmembrane serinethreonine kinase. In one family, we identified a T599 --> A mutation changing an isoleucine into a lysine residue (I200K) within the glycine/serine (GS) domain of BMPR1B, a region involved in phosphorylation of the receptor. In the other family we identified a C1456 --> T mutation leading to an arginine-to-tryptophan amino acid change (R486W) in a highly conserved region C-terminal of the BMPR1B kinase domain. An in vitro kinase assay showed that the I200K mutation is kinase-deficient, whereas the R486W mutation has normal kinase activity, indicating a different pathogenic mechanism. Functional analyses with a micromass culture system revealed a strong inhibition of chondrogenesis by both mutant receptors. Overexpression of mutant chBmpR1b in vivo in chick embryos by using a retroviral system resulted either in a BD phenotype with shortening and/or missing phalanges similar to the human phenotype or in severe hypoplasia of the entire limb. These findings imply that both mutations identified in human BMPR1B affect cartilage formation in a dominant-negative manner.
Figures
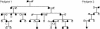


References
-
- Bell, J. (1951) in Treasury of Human Inheritance (Cambridge Univ. Press, London), Vol. 5, pp. 1–31.
-
- Mohr, O. L. & Wried, C. (1919) A New Type of Hereditary Brachphalangy in Man (Institution of Washington, Washington, DC).
-
- Gao, B., Guo, J., She, C., Shu, A., Yang, M., Tan, Z., Yang, X., Guo, S., Feng, G. & He, L. (2001) Nat. Genet. 28, 386–388. - PubMed
-
- Oldridge, M., Fortuna, A. M., Maringa, M., Propping, P., Mansour, S., Pollit, C., DeChiara, T. M., Kimble, R. B., Valenzuela, D. M., Yancopoulos, G. D. & Wilkie, A. O. (2000) Nat. Genet. 24, 275–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

