Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1)
- PMID: 14523239
- PMCID: PMC218783
- DOI: 10.1073/pnas.2032100100
Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1)
Abstract
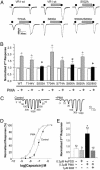
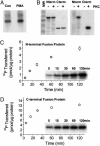
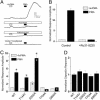
Protein kinase C (PKC) modulates the function of the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). This modulation manifests as increased current when the channel is activated by capsaicin. In addition, studies have suggested that phosphorylation by PKC might directly gate the channel, because PKC-activating phorbol esters induce TRPV1 currents in the absence of applied ligands. To test whether PKC both modulates and gates the TRPV1 function by direct phosphorylation, we used direct sequencing to determine the major sites of PKC phosphorylation on TRPV1 intracellular domains. We then tested the ability of the PKC-activating phorbol 12-myristate 13-acetate (PMA) to potentiate capsaicin-induced currents and to directly gate TRPV1. We found that mutation of S800 to alanine significantly reduced the PMA-induced enhancement of capsaicin-evoked currents and the direct activation of TRPV1 by PMA. Mutation of S502 to alanine reduced PMA enhancement of capsaicin-evoked currents, but had no effect on direct activation of TRPV1 by PMA. Conversely, mutation of T704 to alanine had no effect on PMA enhancement of capsaicin-evoked currents but dramatically reduced direct activation of TRPV1 by PMA. These results, combined with pharmacological studies showing that inactive phorbol esters also weakly activate TRPV1, suggest that PKC-mediated phosphorylation modulates TRPV1 but does not directly gate the channel. Rather, currents induced by phorbol esters result from the combination of a weak direct ligand-like activation of TRPV1 and the phosphorylation-induced enhancement of the TRPV1 function. Furthermore, modulation of the TRPV1 function by PKC appears to involve distinct phosphorylation sites depending on the mechanism of channel activation.
Figures


References
-
- Barber, L. A. & Vasko, M. R. (1996) J. Neurochem. 67, 72-80. - PubMed
-
- Leng, S., Mizumura, K., Koda, H. & Kumazawa, T. (1996) Neurosci. Lett. 206, 13-16. - PubMed
-
- Schepelmann, K., Messlinger, K. & Schmidt, R. F. (1993) Exp. Brain Res. 92, 391-398. - PubMed
-
- Ahlgren, S. C. & Levine, J. D. (1994) J. Neurophysiol. 72, 684-692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

