Invasion-inhibitory antibodies inhibit proteolytic processing of apical membrane antigen 1 of Plasmodium falciparum merozoites
- PMID: 14526103
- PMCID: PMC218752
- DOI: 10.1073/pnas.2032858100
Invasion-inhibitory antibodies inhibit proteolytic processing of apical membrane antigen 1 of Plasmodium falciparum merozoites
Abstract
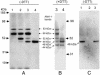
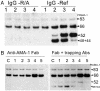
Apical membrane antigen 1 (AMA-1) is a promising vaccine candidate for Plasmodium falciparum malaria. Antibodies against AMA-1 of P. falciparum (PfAMA-1) interrupt merozoite invasion into RBCs. Initially localized within the apical complex, PfAMA-1 is proteolytically processed and redistributed circumferentially on merozoites at about the time of their release and invasion into RBCs. An 83-kDa precursor form of PfAMA-1 is processed to 66-kDa and then to 48- and 44-kDa products. We show that, even at low concentrations, IgG antibodies against correctly folded recombinant PfAMA-1 cross-linked and trapped the 52-, 48-, and 44-kDa proteolytic products on merozoites. These products are normally shed into the culture medium. At higher concentrations antibodies inhibited invasion into RBCs and caused a reduction in the amount of 44- and 48-kDa products, both on merozoites and in the culture medium. A corresponding increase also occurred in the amount of the 66- and 52-kDa forms detected on the merozoites. These antibodies also prevented circumferential redistribution of AMA-1. In contrast, monovalent invasion-inhibitory Fab fragments caused accumulation of 66- and 52-kDa forms, with no cross-linking, trapping, or prevention of redistribution. Antibodies at low concentrations can be used as trapping agents for intermediate and soluble forms of AMA-1 and are useful for studying proteolytic processing of AMA-1. With this technique, it was confirmed that protease inhibitor chymostatin and Ca2+ chelators can inhibit the breakdown of the 66-kDa form. We propose that antibodies to AMA-1 capable of inhibiting erythrocyte invasion act by disrupting proteolytic processing of AMA-1.
Figures
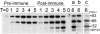


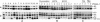
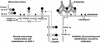
 S—) connecting the 44-kDa form to its proteolytic fragment (see ref. 11). Arrows indicate the substrate (start) and products (end) of each putative enzymatic step. Dashed arrow represents translocation of PfAMA-166 from the apical complex to the surface (apical end). Our data suggest that PfAMA-1 processing on merozoites includes a 52-kDa form, which is either a normal intermediate or a product of anomalous processing that may further be processed to PfAMA-148+44. In this hypothetical model, proteolytic steps 1 and 2 are expected to be chymostatin-sensitive, whereas steps 2 and 3 are EGTA-sensitive. All three proteolytic steps and the redistribution of AMA-1 appear to be sensitive to anti-AMA-1 antibodies. (Left) Normal processing, translocation, and redistribution of AMA-1. (Right) Antibody-mediated processing inhibition, cross-linking, and trapping of AMA-1 fragments.
S—) connecting the 44-kDa form to its proteolytic fragment (see ref. 11). Arrows indicate the substrate (start) and products (end) of each putative enzymatic step. Dashed arrow represents translocation of PfAMA-166 from the apical complex to the surface (apical end). Our data suggest that PfAMA-1 processing on merozoites includes a 52-kDa form, which is either a normal intermediate or a product of anomalous processing that may further be processed to PfAMA-148+44. In this hypothetical model, proteolytic steps 1 and 2 are expected to be chymostatin-sensitive, whereas steps 2 and 3 are EGTA-sensitive. All three proteolytic steps and the redistribution of AMA-1 appear to be sensitive to anti-AMA-1 antibodies. (Left) Normal processing, translocation, and redistribution of AMA-1. (Right) Antibody-mediated processing inhibition, cross-linking, and trapping of AMA-1 fragments.References
-
- Dunn, B. M., ed. (1991) Proteases of Infectious Agents (Academic, New York).
-
- Blackman, M. J. (2000) Curr. Drug Targets 1, 59–83. - PubMed
-
- Holder, A. A., Guevara Patino, J. A., Uthaipibull, C., Syed, S. E., Ling, I. T., Scott-Finnigan, T. & Blackman, M. J. (1999) Parassitologia (Rome) 41, 409–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

