Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control
- PMID: 14526117
- PMCID: PMC281606
- DOI: 10.1104/pp.103.025122
Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control
Abstract
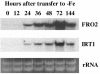
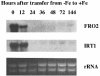
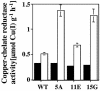
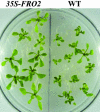
The Arabidopsis FRO2 gene encodes the low-iron-inducible ferric chelate reductase responsible for reduction of iron at the root surface. Here, we report that FRO2 and IRT1, the major transporter responsible for high-affinity iron uptake from the soil, are coordinately regulated at both the transcriptional and posttranscriptional levels. FRO2 and IRT1 are induced together following the imposition of iron starvation and are coordinately repressed following iron resupply. Steady-state mRNA levels of FRO2 and IRT1 are also coordinately regulated by zinc and cadmium. Like IRT1, FRO2 mRNA is detected in the epidermal cells of roots, consistent with its proposed role in iron uptake from the soil. FRO2 mRNA is detected at high levels in the roots and shoots of 35S-FRO2 transgenic plants. However, ferric chelate reductase activity is only elevated in the 35S-FRO2 plants under conditions of iron deficiency, indicating that FRO2 is subject to posttranscriptional regulation, as shown previously for IRT1. Finally, the 35S-FRO2 plants grow better on low iron as compared with wild-type plants, supporting the idea that reduction of ferric iron to ferrous iron is the rate-limiting step in iron uptake.
Figures


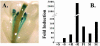


References
-
- Briat J-F, Lobréaux S (1997) Iron transport and storage in plants. Trends Plant Sci 2: 187–193
-
- Brüggemann W, Maas-Kantel K, Moog PR (1993) Iron uptake by leaf mesophyll cells: the role of the plasma membrane-bound ferric-chelate reductase. Planta 190: 151–155
-
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 - PubMed
-
- de la Guardia MD, Alcantara E (1996) Ferric chelate reduction by sunflower (Helianthus annuus L.) leaves: influence of light, oxygen, iron-deficiency and leaf age. J Exp Bot 47: 669–675
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

