Involvement of fatty acid pathways and cortical interaction of the pronuclear complex in Caenorhabditis elegans embryonic polarity
- PMID: 14527340
- PMCID: PMC270048
- DOI: 10.1186/1471-213X-3-8
Involvement of fatty acid pathways and cortical interaction of the pronuclear complex in Caenorhabditis elegans embryonic polarity
Abstract
Background: Cell polarity is essential for many decisions made during development. While investigation of polarity-specific factors has yielded great insights into the polarization process, little is known on how these polarity-specific factors link to the basic cellular mechanisms that function in non-polarity aspects of the cell. To better understand the mechanisms that establish embryonic polarity, we investigated genes required for polarity in the one-cell C. elegans embryo that are also required for other non-polarity functions. This has led to the identification of the Pod-class of mutants that are characterized by osmosensitive embryos and defects in anterior-posterior polarity.
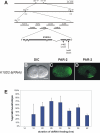
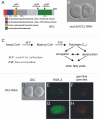
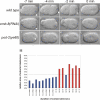
Results: Mutation in either of two loci of this class, emb-8 and pod-2, disrupts embryonic polarization and results in osmotically-sensitive embryos. Loss of emb-8, a previously uncharacterized polarity gene, causes mislocalization of PAR-3 and PAR-2 that molecularly mark the anterior and posterior cortices. emb-8 encodes NADPH-cytochrome P450 reductase, a protein supplying electrons to cytochrome P450-family enzymes, some of which catalyze fatty acid modifications. Cloning of the previously characterized polarity gene pod-2 reveals it encodes acetyl-CoA carboxylase, an enzyme that catalyzes the first step in de novo fatty acid synthesis. Depletion of fatty acid synthase, the next enzyme in the biosynthetic pathway, by RNA-interference (RNAi) also causes similar loss of one-cell polarity. Furthermore, pod-2 polarity defects can be rescued by addition of exogenous fatty acids. By following the behavior of the pronucleus in emb-8 and pod-2 mutant embryos, we demonstrate that loss of polarity correlates with impaired interaction between the pronucleus-centrosome complex and the posterior cortex.
Conclusions: The characterization of emb-8 and pod-2 mutant embryos suggests that the pronucleus-centrosome complex interaction with the cortex plays a direct role in establishing polarity and that fatty acid pathways are important for this polarizing event.
Figures

References
-
- Horvitz HR, Herskowitz I. Mechanisms of asymmetric cell division: two Bs or not two Bs, that is the question. Cell. 1992;68:237–55. - PubMed
-
- Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

