SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis
- PMID: 14527997
- PMCID: PMC218775
- DOI: 10.1073/pnas.2133653100
SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis
Abstract
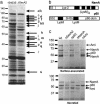
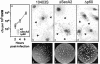
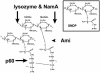
Pathogenic bacteria secrete proteins that promote invasion of host tissues and resistance to immune responses. However, secretion mechanisms that contribute to the enormous morbidity and mortality of Gram-positive bacteria are largely undefined. An auxiliary protein secretion system (SecA2) has recently emerged in Listeria monocytogenes and eight other Gram-positive pathogens. Here, a proteomics approach identified seventeen SecA2-dependent secreted and surface proteins of L. monocytogenes, the two most abundant of which [the p60 and N-acetylmuramidase (NamA) autolysins] hydrolyze bacterial peptidoglycan (PGN) and contribute to host colonization. SecA2-deficient (DeltaSecA2) bacteria were rapidly cleared after systemic infection of murine hosts, and in cultured cells showed reduced cell-cell spread. p60 or NamA deficiencies (Deltap60 and DeltaNamA) caused intermediate reductions in bacterial virulence in vivo, yet showed no defect for infection of cultured cells. Restoration of virulence in Deltap60 bacteria required full-length p60 with an intact catalytic domain, suggesting that PGN hydrolysis by p60 is crucial for L. monocytogenes virulence. Coordinated PGN hydrolysis by p60 and NamA activities is predicted to generate a muramyl glycopeptide, glucosaminylmuramyl dipeptide (GMDP), which is known to modify host inflammatory responses. Thus, SecA2-dependent secretion may promote release of muramyl peptides that subvert host pattern recognition.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

