Targeting of a tropomyosin isoform to short microfilaments associated with the Golgi complex
- PMID: 14528022
- PMCID: PMC307546
- DOI: 10.1091/mbc.e03-03-0176
Targeting of a tropomyosin isoform to short microfilaments associated with the Golgi complex
Abstract
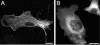
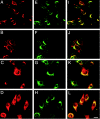
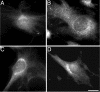
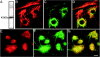
A growing body of evidence suggests that the Golgi complex contains an actin-based filament system. We have previously reported that one or more isoforms from the tropomyosin gene Tm5NM (also known as gamma-Tm), but not from either the alpha- or beta-Tm genes, are associated with Golgi-derived vesicles (Heimann et al., (1999). J. Biol. Chem. 274, 10743-10750). We now show that Tm5NM-2 is sorted specifically to the Golgi complex, whereas Tm5NM-1, which differs by a single alternatively spliced internal exon, is incorporated into stress fibers. Tm5NM-2 is localized to the Golgi complex consistently throughout the G1 phase of the cell cycle and it associates with Golgi membranes in a brefeldin A-sensitive and cytochalasin D-resistant manner. An actin antibody, which preferentially reacts with the ends of microfilaments, newly reveals a population of short actin filaments associated with the Golgi complex and particularly with Golgi-derived vesicles. Tm5NM-2 is also found on these short microfilaments. We conclude that an alternative splice choice can restrict the sorting of a tropomyosin isoform to short actin filaments associated with Golgi-derived vesicles. Our evidence points to a role for these Golgi-associated microfilaments in vesicle budding at the level of the Golgi complex.
Figures






Similar articles
-
Sorting of tropomyosin isoforms in synchronised NIH 3T3 fibroblasts: evidence for distinct microfilament populations.Cell Motil Cytoskeleton. 2000 Nov;47(3):189-208. doi: 10.1002/1097-0169(200011)47:3<189::AID-CM3>3.0.CO;2-C. Cell Motil Cytoskeleton. 2000. PMID: 11056521
-
Actin microfilaments facilitate the retrograde transport from the Golgi complex to the endoplasmic reticulum in mammalian cells.Traffic. 2001 Oct;2(10):717-26. doi: 10.1034/j.1600-0854.2001.21006.x. Traffic. 2001. PMID: 11576448
-
Tropomyosin - master regulator of actin filament function in the cytoskeleton.J Cell Sci. 2015 Aug 15;128(16):2965-74. doi: 10.1242/jcs.172502. Epub 2015 Aug 3. J Cell Sci. 2015. PMID: 26240174 Review.
-
ER/Golgi trafficking is facilitated by unbranched actin filaments containing Tpm4.2.Cytoskeleton (Hoboken). 2017 Oct;74(10):379-389. doi: 10.1002/cm.21405. Epub 2017 Aug 31. Cytoskeleton (Hoboken). 2017. PMID: 28834398 Free PMC article.
-
Tropomyosin isoforms: divining rods for actin cytoskeleton function.Trends Cell Biol. 2005 Jun;15(6):333-41. doi: 10.1016/j.tcb.2005.04.007. Trends Cell Biol. 2005. PMID: 15953552 Review.
Cited by
-
Involvement of the Rho-mDia1 pathway in the regulation of Golgi complex architecture and dynamics.Mol Biol Cell. 2011 Aug 15;22(16):2900-11. doi: 10.1091/mbc.E11-01-0007. Epub 2011 Jun 16. Mol Biol Cell. 2011. PMID: 21680709 Free PMC article.
-
Intersectin-1 interacts with the golgin GCC88 to couple the actin network and Golgi architecture.Mol Biol Cell. 2019 Feb 1;30(3):370-386. doi: 10.1091/mbc.E18-05-0313. Epub 2018 Dec 12. Mol Biol Cell. 2019. PMID: 30540523 Free PMC article.
-
Actin dynamics coupled to clathrin-coated vesicle formation at the trans-Golgi network.J Cell Biol. 2004 Jun 21;165(6):781-8. doi: 10.1083/jcb.200403120. J Cell Biol. 2004. PMID: 15210728 Free PMC article.
-
High molecular weight tropomyosins regulate osteoclast cytoskeletal morphology.Bone. 2008 Nov;43(5):951-60. doi: 10.1016/j.bone.2008.06.017. Epub 2008 Jul 12. Bone. 2008. PMID: 18674650 Free PMC article.
-
Regulation of actin polymerization by tropomodulin-3 controls megakaryocyte actin organization and platelet biogenesis.Blood. 2015 Jul 23;126(4):520-30. doi: 10.1182/blood-2014-09-601484. Epub 2015 May 11. Blood. 2015. PMID: 25964668 Free PMC article.
References
-
- Adami, R., Cintio, O., Trombelta, G., Choquet, D., and Grazi, E. (2002). Effects of chemical modification tropomyosin, and myosin subfragment 1 on the yield strength and critical concentration of F-actin. Biochemistry 41, 5907-5912. - PubMed
-
- Bamburg, J.R. (1999). Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 15, 185-230. - PubMed
-
- Beck, K.A., Buchanan, J.A., and Nelson, W.J. (1997). Golgi membrane skeleton: identification, localization and oligomerization of a 195 kDa ankyrin associated with the Golgi complex. J. Cell Sci. 110, 1239-1249. - PubMed
-
- Blanchoin, L., Pollard, T.D., and Hitchcock-DeGregori, S.E. (2001). Inhibition of the Arp2/3 complex-nucleated actin polymerization and branch formation by tropomyosin. Curr. Biol. 11, 1300-1304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

