A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen
- PMID: 14528023
- PMCID: PMC284788
- DOI: 10.1091/mbc.e02-11-0731
A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen
Abstract
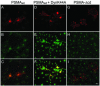
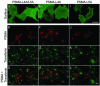
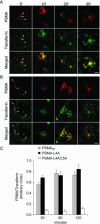
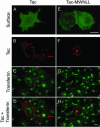
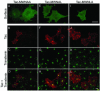
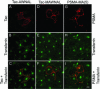
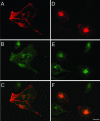
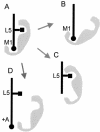
Prostate-specific membrane antigen (PSMA) is a transmembrane protein expressed at high levels in prostate cancer and in tumor-associated neovasculature. In this study, we report that PSMA is internalized via a clathrin-dependent endocytic mechanism and that internalization of PSMA is mediated by the five N-terminal amino acids (MWNLL) present in its cytoplasmic tail. Deletion of the cytoplasmic tail abolished PSMA internalization. Mutagenesis of N-terminal amino acid residues at position 2, 3, or 4 to alanine did not affect internalization of PSMA, whereas mutation of amino acid residues 1 or 5 to alanine strongly inhibited internalization. Using a chimeric protein composed of Tac antigen, the alpha-chain of interleukin 2-receptor, fused to the first five amino acids of PSMA (Tac-MWNLL), we found that this sequence is sufficient for PSMA internalization. In addition, inclusion of additional alanines into the MWNLL sequence either in the Tac chimera or the full-length PSMA strongly inhibited internalization. From these results, we suggest that a novel MXXXL motif in the cytoplasmic tail mediates PSMA internalization. We also show that dominant negative micro2 of the adaptor protein (AP)-2 complex strongly inhibits the internalization of PSMA, indicating that AP-2 is involved in the internalization of PSMA mediated by the MXXXL motif.
Figures

Similar articles
-
Interaction of prostate specific membrane antigen with clathrin and the adaptor protein complex-2.Int J Oncol. 2007 Nov;31(5):1199-203. Int J Oncol. 2007. PMID: 17912448
-
Cloning and characterization of canine prostate-specific membrane antigen.Prostate. 2013 May;73(6):642-50. doi: 10.1002/pros.22605. Epub 2013 Jan 28. Prostate. 2013. PMID: 23359458
-
AP-2-dependent internalization of potassium channel Kir2.3 is driven by a novel di-hydrophobic signal.J Biol Chem. 2008 Mar 7;283(10):5973-84. doi: 10.1074/jbc.M709756200. Epub 2008 Jan 7. J Biol Chem. 2008. PMID: 18180291
-
Endocytosis of the glucose transporter GLUT8 is mediated by interaction of a dileucine motif with the beta2-adaptin subunit of the AP-2 adaptor complex.J Cell Sci. 2006 Jun 1;119(Pt 11):2321-31. doi: 10.1242/jcs.02943. J Cell Sci. 2006. PMID: 16723738
-
Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer.J Cell Biochem. 2004 Feb 15;91(3):528-39. doi: 10.1002/jcb.10661. J Cell Biochem. 2004. PMID: 14755683 Review.
Cited by
-
Discriminatory Role of Detergent-Resistant Membranes in the Dimerization and Endocytosis of Prostate-Specific Membrane Antigen.PLoS One. 2013 Jun 19;8(6):e66193. doi: 10.1371/journal.pone.0066193. Print 2013. PLoS One. 2013. PMID: 23840421 Free PMC article.
-
A novel cytoplasmic tail motif regulates mouse corin expression on the cell surface.Biochem Biophys Res Commun. 2015 Sep 11;465(1):152-8. doi: 10.1016/j.bbrc.2015.07.156. Epub 2015 Aug 1. Biochem Biophys Res Commun. 2015. PMID: 26241673 Free PMC article.
-
Development of 5D3-DM1: A Novel Anti-Prostate-Specific Membrane Antigen Antibody-Drug Conjugate for PSMA-Positive Prostate Cancer Therapy.Mol Pharm. 2020 Sep 8;17(9):3392-3402. doi: 10.1021/acs.molpharmaceut.0c00457. Epub 2020 Aug 17. Mol Pharm. 2020. PMID: 32803984 Free PMC article.
-
Crystal structure of prostate-specific membrane antigen, a tumor marker and peptidase.Proc Natl Acad Sci U S A. 2005 Apr 26;102(17):5981-6. doi: 10.1073/pnas.0502101102. Epub 2005 Apr 18. Proc Natl Acad Sci U S A. 2005. PMID: 15837926 Free PMC article.
-
68Ga-PSMA PET/CT imaging in recurrent prostate cancer: Where are we now?Cent European J Urol. 2017;70(1):37-43. doi: 10.5173/ceju.2017.947. Epub 2017 Jan 11. Cent European J Urol. 2017. PMID: 28461986 Free PMC article. Review.
References
-
- Anilkumar, G., Rajasekaran, S.A., Wang, S., Hankinson, O., Bander, N.H., and Rajasekaran, A.K. (2003). Prostate-specific membrane antigen association with filamin A modulates its internalization and NAALADase activity. Cancer Res. 63, 2645–2648. - PubMed
-
- Bello, V., Goding, J.W., Greengrass, V., Sali, A., Dubljevic, V., Lenoir, C., Trugnan, G., and Maurice, M. (2001). Characterization of a di-leucine-based signal in the cytoplasmic tail of the nucleotide-pyrophosphatase NPP1 that mediates basolateral targeting but not endocytosis. Mol. Biol. Cell 12, 3004–3015. - PMC - PubMed
-
- Boll, W., Rapoport, I., Brunner, C., Modis, Y., Prehn, S., and Kirchhausen, T. (2002). The mu2 subunit of the clathrin adaptor AP-2 binds to FDNPVY and YppO sorting signals at distinct sites. Traffic 3, 590–600. - PubMed
-
- Bonifacino, J.S., and Traub, L.M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

