Histamine induces cytoskeletal changes in human eosinophils via the H(4) receptor
- PMID: 14530216
- PMCID: PMC1574117
- DOI: 10.1038/sj.bjp.0705530
Histamine induces cytoskeletal changes in human eosinophils via the H(4) receptor
Abstract
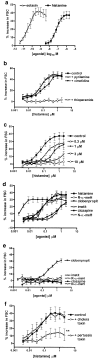
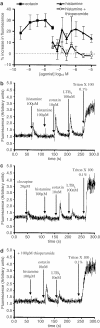
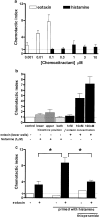
1. Histamine (0.004-2 microm) induced a concentration-dependent shape change of human eosinophils, but not of neutrophils or basophils, detected as an increase in forward scatter (FSC) in the gated autofluorescence/forward scatter (GAFS) assay. 2. The histamine-induced eosinophil shape change was completely abolished by thioperamide (10 microm), an H3/H4 receptor antagonist, but was not inhibited by pyrilamine or cimetidine (10 microm), H1 and H2 receptor antagonists, respectively. The H4 receptor agonists, clobenpropit and clozapine (0.004-2 microm), which are also H3 receptor antagonists, both induced eosinophil shape change, which was inhibited by thioperamide (10 microm). The H3/H4 receptor agonists, imetit, R-alpha-methyl histamine and N-alpha-methyl histamine (0.004-2 microm) also induced eosinophil shape change. 3. Histamine induced actin polymerisation (0.015-10 microm), intracellular calcium mobilisation (10-100 microm) and a significant upregulation of expression of the cell adhesion molecule CD11b (0.004-10 microm) in eosinophils, all of which were inhibited by thioperamide (10-100 microm). In addition, the H4 receptor agonist/H3 receptor antagonist clozapine (20 microm) stimulated a rise in intracellular calcium in eosinophils. 4. Activation of H4 receptors by histamine (1 microm) primed eosinophils for increased chemotactic responses to eotaxin, but histamine (0.1-10 microm) did not directly induce chemotaxis of eosinophils. 5. Pertussis toxin (1 microg ml-1) inhibited shape change and actin polymerisation responses induced by histamine showing that these effects are mediated by coupling to a Galphai/o G-protein. 6. This study demonstrates that human eosinophils express functional H4 receptors and may provide a novel target for allergic disease therapy.
Figures



Comment in
-
Histamine H4 antagonism: a therapy for chronic allergy?Br J Pharmacol. 2004 May;142(1):5-7. doi: 10.1038/sj.bjp.0705730. Br J Pharmacol. 2004. PMID: 15130999 Free PMC article. Review. No abstract available.
References
-
- ARRANG J.M., GARBARG M., LANCELOT J.C., LECOMTE J.M., POLLARD H., ROBBA M., SCHUNACK W., SCHWARTZ J.C. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987;327:117–123. - PubMed
-
- ARRANG J.M., GARBARG M., SCHWARTZ J.C. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. - PubMed
-
- BISSONNETTE E.Y. Histamine inhibits tumor necrosis factor alpha release by mast cells through H2 and H3 receptors. Am. J. Respir. Cell Mol. Biol. 1996;14:620–626. - PubMed
-
- BOUSQUET J., CHANEZ P., LACOSTE J.Y., BARNEON G., GHAVANIAN N., ENANDER I., VENGE P., AHLSTEDT S., SIMONY-LAFONTAINE J., GODARD P. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990;323:1033–1039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

