The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells
- PMID: 14530379
- PMCID: PMC2194218
- DOI: 10.1084/jem.20030913
The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells
Abstract
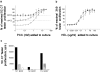
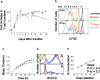
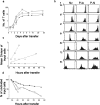
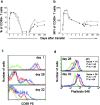
The quantitative adaptation of receptor thresholds allows cells to tailor their responses to changes in ambient ligand concentration in many biological systems. Such a cell-intrinsic calibration of T cell receptor (TCR) sensitivity could be involved in regulating responses to autoantigens, but this has never been demonstrated for peripheral T cells. We examined the ability of monoclonal naive T cells to modulate their responsiveness differentially after exposure to fourfold different levels of persistent antigen stimulation in vivo. T cells expanded and entered a tolerant state with different kinetics in response to the two levels of stimulation, but eventually adjusted to a similar slow rate of turnover. In vivo restimulation revealed a greater impairment in the proliferative ability of T cells resident in a higher antigen presentation environment. We also observed subtle differences in TCR signaling and in vitro cytokine production consistent with differential adaptation. Unexpectedly, the system failed to similarly compensate to the persistent stimulus in vivo at the level of CD69 expression and actin polymerization. This greater responsiveness of T cells residing in a host with a lower level of antigen presentation allows us to demonstrate for the first time an intrinsic tuning process in mature T lymphocytes, albeit one more complex than current theories predict.
Figures


References
-
- Sklar, L.A., and G.M. Omann. 1990. Kinetics and amplification in neutrophil activation and adaptation. Semin. Cell Biol. 1:115–123. - PubMed
-
- Sentman, C.L., M.Y. Olsson, and K. Karre. 1995. Missing self recognition by natural killer cells in MHC class I transgenic mice. A ‘receptor calibration’ model for how effector cells adapt to self. Semin. Immunol. 7:109–119. - PubMed
-
- Lucas, B., I. Stefanova, K. Yasutomo, N. Dautigny, and R.N. Germain. 1999. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 10:367–376. - PubMed
-
- Azzam, H.S., J.B. DeJarnette, K. Huang, R. Emmons, C.S. Park, C.L. Sommers, D. El Khoury, E.W. Shores, and P.E. Love. 2001. Fine tuning of TCR signaling by CD5. J. Immunol. 166:5464–5472. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

