Y box-binding protein 1 induces resistance to oncogenic transformation by the phosphatidylinositol 3-kinase pathway
- PMID: 14530393
- PMCID: PMC218767
- DOI: 10.1073/pnas.2135336100
Y box-binding protein 1 induces resistance to oncogenic transformation by the phosphatidylinositol 3-kinase pathway
Abstract
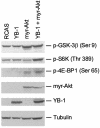
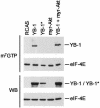
Y box-binding protein 1 (YB-1) is a multifunctional protein that can act as a regulator of transcription and of translation. In chicken embryo fibroblasts transformed by the oncoproteins P3k (phosphatidylinositol 3-kinase) or Akt, YB-1 is transcriptionally down-regulated. Expression of YB-1 from a retroviral vector induces a strong cellular resistance to transformation by P3k or Akt but does not affect sensitivity to transformation by other oncoproteins, such as Src, Jun, or Qin. The YB-1-expressing cells assume a tightly adherent, flat phenotype, with YB-1 localized in the cytoplasm, and show a greatly reduced saturation density. Both cap-dependent and cap-independent translation is inhibited in these cells, but the activity of Akt remains unaffected, suggesting that YB-1 functions downstream of Akt. A YB-1 protein with a loss-of-function mutation in the RNA-binding motif no longer binds to the mRNA cap structure, is localized in the cell nucleus, does not induce the flat cellular phenotype, and fails to interfere with P3k- or Akt-induced oncogenic transformation. This mutant also does not inhibit cap-dependent or cap-independent translation. These results suggest that YB-1 acts like a rapamycin mimic, inhibiting translational events that are required in phosphatidylinositol 3-kinase-driven oncogenic transformation.
Figures
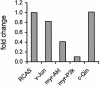



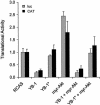
 ); CAT activity represents cap-dependent translation (▪). Experiments were carried out three times; standard deviations are shown as error bars. The translational activity of cells transfected with the empty vector was designated 1.0.
); CAT activity represents cap-dependent translation (▪). Experiments were carried out three times; standard deviations are shown as error bars. The translational activity of cells transfected with the empty vector was designated 1.0.References
-
- Bellacosa, A., Testa, J. R., Staal, S. P. & Tsichlis, P. N. (1991) Science 254, 274-277. - PubMed
-
- Chang, H. W., Aoki, M., Fruman, D., Auger, K. R., Bellacosa, A., Tsichlis, P. N., Cantley, L. C., Roberts, T. M. & Vogt, P. K. (1997) Science 276, 1848-1850. - PubMed
-
- Alessi, D. R. & Downes, C. P. (1998) Biochim. Biophys. Acta 1436, 151-164. - PubMed
-
- Wymann, M. P. & Pirola, L. (1998) Biochim. Biophys. Acta 1436, 127-150. - PubMed
-
- Scheid, M. P. & Woodgett, J. R. (2001) Nat. Rev. Mol. Cell Biol. 2, 760-768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

