Ewing sarcoma/primitive neuroectodermal tumor of the chest wall: impact of initial versus delayed resection on tumor margins, survival, and use of radiation therapy
- PMID: 14530727
- PMCID: PMC1360114
- DOI: 10.1097/01.sla.0000089857.45191.52
Ewing sarcoma/primitive neuroectodermal tumor of the chest wall: impact of initial versus delayed resection on tumor margins, survival, and use of radiation therapy
Abstract
Objective: To establish outcome and optimal timing of local control for patients with nonmetastatic Ewing sarcoma/primitive neuroectodermal tumor (ES/PNET) of the chest wall.
Methods: Patients < or =30 years of age with ES/PNET of the chest wall were entered in 2 consecutive protocols. Therapy included multiagent chemotherapy; local control was achieved by resection, radiotherapy, or both. We compared completeness of resection and disease-free survival in patients undergoing initial surgical resection versus those treated with neoadjuvant chemotherapy followed by resection, radiotherapy, or both. Patients with a positive surgical margin received radiotherapy.
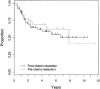
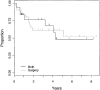
Results: Ninety-eight (11.3%) of 869 patients had primary tumors of the chest wall. Median follow-up was 3.47 years and 5-year event-free survival was 56% for the chest wall lesions. Ten of 20 (50%) initial resections resulted in negative margins compared with 41 of 53 (77%) negative margins with delayed resections after chemotherapy (P = 0.043). Event-free survival did not differ by timing of surgery (P = 0.69) or type of local control (P = 0.17). Initial chemotherapy decreased the percentage of patients needing radiation therapy. Seventeen of 24 patients (70.8%) with initial surgery received radiotherapy compared with 34 of 71 patients (47.9%) who started with chemotherapy (P = 0.061). If a delayed operation was performed, excluding those patients who received only radiotherapy for local control, only 25 of 62 patients needed radiotherapy (40.3%; P = 0.016).
Conclusion: The likelihood of complete tumor resection with a negative microscopic margin and consequent avoidance of external beam radiation and its potential complications is increased with neoadjuvant chemotherapy and delayed resection of chest wall ES/PNET.
Figures


References
-
- Strong LC, Herson J, Osborne BM, et al. Risk of radiation-related subsequent malignant tumors in survivors of Ewing’s sarcoma. J Natl Cancer Inst. 1979;62:1401–1406. - PubMed
-
- Tucker MA, D’Angio GJ, Boice JD Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. - PubMed
-
- Kuttesch JF Jr, Wexler LH, Marcus RB, et al. Second malignancies after Ewing’s sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14:2818–2825. - PubMed
-
- Paulussen M, Ahrens S, Lehnert M, et al. Second malignancies after Ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. Ann Oncol. 2001;12:1619–1630. - PubMed
-
- Shamberger RC, Laquaglia MP, Krailo MD, et al. Ewing sarcoma of the rib: results of an intergroup study with analysis of outcome by timing of resection. J Thorac Cardiovasc Surg. 2000;119:1154–1161. - PubMed
Publication types
MeSH terms
Grants and funding
- CA-33587/CA/NCI NIH HHS/United States
- CA-17829/CA/NCI NIH HHS/United States
- CA-10198/CA/NCI NIH HHS/United States
- CA-36015/CA/NCI NIH HHS/United States
- CA-03888/CA/NCI NIH HHS/United States
- CA-28439/CA/NCI NIH HHS/United States
- CA-20549/CA/NCI NIH HHS/United States
- CA-53128/CA/NCI NIH HHS/United States
- CA-07306/CA/NCI NIH HHS/United States
- CA-33625/CA/NCI NIH HHS/United States
- CA-29691/CA/NCI NIH HHS/United States
- CA-31566/CA/NCI NIH HHS/United States
- CA-26044/CA/NCI NIH HHS/United States
- CA-20320/CA/NCI NIH HHS/United States
- CA-69428/CA/NCI NIH HHS/United States
- CA-28383/CA/NCI NIH HHS/United States
- CA-11796/CA/NCI NIH HHS/United States
- CA-28476/CA/NCI NIH HHS/United States
- CA-02971/CA/NCI NIH HHS/United States
- CA-25408/CA/NCI NIH HHS/United States
- CA-15525/CA/NCI NIH HHS/United States
- CA-29013/CA/NCI NIH HHS/United States
- CA-29233/CA/NCI NIH HHS/United States
- CA-26126/CA/NCI NIH HHS/United States
- CA-26270/CA/NCI NIH HHS/United States
- CA-14560/CA/NCI NIH HHS/United States
- CA-045587/CA/NCI NIH HHS/United States
- CA-32053/CA/NCI NIH HHS/United States
- CA-05587/CA/NCI NIH HHS/United States
- CA-03526/CA/NCI NIH HHS/United States
- CA-30969/CA/NCI NIH HHS/United States
- CA-28851/CA/NCI NIH HHS/United States
- CA-33603/CA/NCI NIH HHS/United States
- CA-29293/CA/NCI NIH HHS/United States
- CA-29314/CA/NCI NIH HHS/United States
- CA-15989/CA/NCI NIH HHS/United States
- CA-02649/CA/NCI NIH HHS/United States
- CA-03161/CA/NCI NIH HHS/United States
- U10 CA029139/CA/NCI NIH HHS/United States
- CA-42764/CA/NCI NIH HHS/United States
- CA-41573/CA/NCI NIH HHS/United States
- CA-03750/CA/NCI NIH HHS/United States
- CA-11233/CA/NCI NIH HHS/United States
- CA-13809/CA/NCI NIH HHS/United States
- CA-05436/CA/NCI NIH HHS/United States
- CA-27678/CA/NCI NIH HHS/United States
- CA-28882/CA/NCI NIH HHS/United States
- CA-69177/CA/NCI NIH HHS/United States
- CA-07431/CA/NCI NIH HHS/United States
- CA-13539/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

