Dual resistance to Bacillus thuringiensis Cry1Ac and Cry2Aa toxins in Heliothis virescens suggests multiple mechanisms of resistance
- PMID: 14532042
- PMCID: PMC201244
- DOI: 10.1128/AEM.69.10.5898-5906.2003
Dual resistance to Bacillus thuringiensis Cry1Ac and Cry2Aa toxins in Heliothis virescens suggests multiple mechanisms of resistance
Abstract
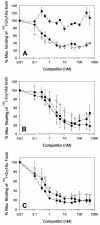
One strategy for delaying evolution of resistance to Bacillus thuringiensis crystal (Cry) endotoxins is the production of multiple Cry toxins in each transgenic plant (gene stacking). This strategy relies upon the assumption that simultaneous evolution of resistance to toxins that have different modes of action will be difficult for insect pests. In B. thuringiensis-transgenic (Bt) cotton, production of both Cry1Ac and Cry2Ab has been proposed to delay resistance of Heliothis virescens (tobacco budworm). After previous laboratory selection with Cry1Ac, H. virescens strains CXC and KCBhyb developed high levels of cross-resistance not only to toxins similar to Cry1Ac but also to Cry2Aa. We studied the role of toxin binding alteration in resistance and cross-resistance with the CXC and KCBhyb strains. In toxin binding experiments, Cry1A and Cry2Aa toxins bound to brush border membrane vesicles from CXC, but binding of Cry1Aa was reduced for the KCBhyb strain compared to susceptible insects. Since Cry1Aa and Cry2Aa do not share binding proteins in H. virescens, our results suggest occurrence of at least two mechanisms of resistance in KCBhyb insects, one of them related to reduction of Cry1Aa toxin binding. Cry1Ac bound irreversibly to brush border membrane vesicles (BBMV) from YDK, CXC, and KCBhyb larvae, suggesting that Cry1Ac insertion was unaffected. These results highlight the genetic potential of H. virescens to become resistant to distinct Cry toxins simultaneously and may question the effectiveness of gene stacking in delaying evolution of resistance.
Figures


Similar articles
-
Cross-resistance responses of CrylAc-selected Heliothis virescens (Lepidoptera: Noctuidae) to the Bacillus thuringiensis protein vip3A.J Econ Entomol. 2007 Feb;100(1):180-6. doi: 10.1603/0022-0493(2007)100[180:crochv]2.0.co;2. J Econ Entomol. 2007. PMID: 17370826
-
Altered Glycosylation of 63- and 68-kilodalton microvillar proteins in Heliothis virescens correlates with reduced Cry1 toxin binding, decreased pore formation, and increased resistance to Bacillus thuringiensis Cry1 toxins.Appl Environ Microbiol. 2002 Nov;68(11):5711-7. doi: 10.1128/AEM.68.11.5711-5717.2002. Appl Environ Microbiol. 2002. PMID: 12406769 Free PMC article.
-
The HevCaLP protein mediates binding specificity of the Cry1A class of Bacillus thuringiensis toxins in Heliothis virescens.Biochemistry. 2004 Nov 9;43(44):14299-305. doi: 10.1021/bi048500i. Biochemistry. 2004. PMID: 15518581
-
Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control.Toxicon. 2007 Mar 15;49(4):423-35. doi: 10.1016/j.toxicon.2006.11.022. Epub 2006 Nov 30. Toxicon. 2007. PMID: 17198720 Free PMC article. Review.
-
Interactions between insecticidal cry toxins and their receptors.Curr Genet. 2025 Mar 29;71(1):9. doi: 10.1007/s00294-025-01312-1. Curr Genet. 2025. PMID: 40156649 Review.
Cited by
-
The Potential Role of the Methionine Aminopeptidase Gene PxMetAP1 in a Cosmopolitan Pest for Bacillus thuringiensis Toxin Tolerance.Int J Mol Sci. 2022 Oct 27;23(21):13005. doi: 10.3390/ijms232113005. Int J Mol Sci. 2022. PMID: 36361800 Free PMC article.
-
Binding sites for Bacillus thuringiensis Cry2Ae toxin on heliothine brush border membrane vesicles are not shared with Cry1A, Cry1F, or Vip3A toxin.Appl Environ Microbiol. 2011 May;77(10):3182-8. doi: 10.1128/AEM.02791-10. Epub 2011 Mar 25. Appl Environ Microbiol. 2011. PMID: 21441333 Free PMC article.
-
Reduced levels of membrane-bound alkaline phosphatase are common to lepidopteran strains resistant to Cry toxins from Bacillus thuringiensis.PLoS One. 2011 Mar 1;6(3):e17606. doi: 10.1371/journal.pone.0017606. PLoS One. 2011. PMID: 21390253 Free PMC article.
-
Resistance of Lepidopteran Pests to Bacillus thuringiensis Toxins: Evidence of Field and Laboratory Evolved Resistance and Cross-Resistance, Mode of Resistance Inheritance, Fitness Costs, Mechanisms Involved and Management Options.Toxins (Basel). 2024 Jul 12;16(7):315. doi: 10.3390/toxins16070315. Toxins (Basel). 2024. PMID: 39057955 Free PMC article. Review.
-
Intestinal regeneration as an insect resistance mechanism to entomopathogenic bacteria.Curr Opin Insect Sci. 2016 Jun;15:104-10. doi: 10.1016/j.cois.2016.04.008. Epub 2016 Apr 20. Curr Opin Insect Sci. 2016. PMID: 27436739 Free PMC article. Review.
References
-
- Adamczyk, J. J., L. C. Adams, and D. D. Hardee. 2001. Field efficacy and seasonal expression profiles for terminal leaves of single and double Bacillus thuringiensis toxin cotton genotypes. J. Econ. Entomol. 94:1589-1593. - PubMed
-
- Betz, F. S., B. G. Hammond, and R. L. Fuchs. 2000. Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul. Toxicol. Pharm. 32:156-173. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Candas, M., O. Loseva, B. Oppert, P. Kosaraju, and L. A. Bulla, Jr. 2003. Insect resistance to Bacillus thuringiensis: alterations in the Indianmeal moth larval gut proteome. Mol. Cell Proteomics 2:19-28. - PubMed
-
- Chiang, A. S., D. F. Yen, and W. K. Peng. 1986. Defense reaction of midgut epithelial cells in the rice moth larva (Corcyra cephalonica) infected with Bacillus thuringiensis. J. Invertebr. Pathol. 47:333-339.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

