EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer
- PMID: 14532106
- PMCID: PMC213796
- DOI: 10.1093/emboj/cdg542
EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer
Erratum in
-
Author Correction: EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer.EMBO J. 2024 Mar;43(5):886. doi: 10.1038/s44318-024-00033-4. EMBO J. 2024. PMID: 38347304 Free PMC article.
Abstract
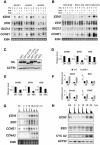
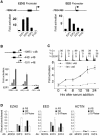
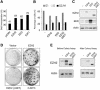
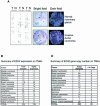
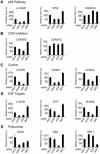
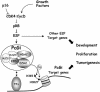
Recent experiments have demonstrated that the Polycomb group (PcG) gene EZH2 is highly expressed in metastatic prostate cancer and in lymphomas. EZH2 is a component of the PRC2 histone methyltransferase complex, which also contains EED and SUZ12 and is required for the silencing of HOX gene expression during embryonic development. Here we demonstrate that both EZH2 and EED are essential for the proliferation of both transformed and non-transformed human cells. In addition, the pRB-E2F pathway tightly regulates their expression and, consistent with this, we find that EZH2 is highly expressed in a large set of human tumors. These results raise the question whether EZH2 is a marker of proliferation or if it is actually contributing to tumor formation. Significantly, we propose that EZH2 is a bona fide oncogene, since we find that ectopic expression of EZH2 is capable of providing a proliferative advantage to primary cells and, in addition, its gene locus is specifically amplified in several primary tumors.
Figures


References
-
- Brock H.W. and van Lohuizen,M. (2001) The Polycomb group—no longer an exclusive club? Curr. Opin. Genet. Dev., 11, 175–181. - PubMed
-
- Cao R., Wang,L., Wang,H., Xia,L., Erjument-Bromage,H., Tempst,P., Jones,R.S. and Zhang,Y. (2002) Role of Histone H3 Lysine 27 methylation in Polycomb-Group silencing. Science, 298, 1039–1043. - PubMed
-
- Cardoso C., Mignon,C., Hetet,G., Grandchamps,B., Fontes M. and Colleaux,I. (2000) The human EZH2 gene: genomic organisation and revised mapping in 7q35 within a critical region of malignant disorders. Eur. J. Hum. Genet., 8, 174–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

