Interaction of fascin and protein kinase Calpha: a novel intersection in cell adhesion and motility
- PMID: 14532112
- PMCID: PMC213775
- DOI: 10.1093/emboj/cdg521
Interaction of fascin and protein kinase Calpha: a novel intersection in cell adhesion and motility
Abstract
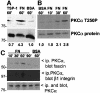
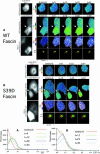
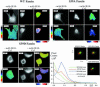
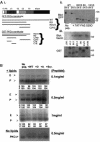
Coordination of protrusive and contractile cell-matrix contacts is important for cell adhesion and migration, but the mechanisms involved are not well understood. We report an unexpected direct association between fascin, an actin-bundling component of filopodia, microspikes and lamellipodial ribs, and protein kinase Calpha (PKCalpha), a regulator of focal adhesions. The association is detectable by protein-protein binding in vitro, by coimmunoprecipitation from cell extracts, and in live cells as fluorescence resonance energy transfer detected by fluorescence imaging lifetime microscopy. The interaction is physiologically regulated by the extracellular matrix context of cells, depends on activation of PKCalpha and is mediated by the C1B domain of PKCalpha. Strikingly, a fascin mutant, fascin S39D, associates constitutively with PKCalpha. Through use of a newly developed set of membrane-permeable peptides that separately inhibit either fascin/PKCalpha or fascin/actin binding, we have uncovered that specific blockade of the fascin/PKCalpha interaction increases cell migration on fibronectin in conjunction with increased fascin protrusions and remodeling of focal adhesions. These results identify the fascin-PKCalpha interaction as an important novel intersection in the regulation and networking of cell-matrix contacts.
Figures



References
-
- Adams J.C. (1995) Formation of stable microspikes containing actin and the 55kDa actin-bundling protein, fascin, is a consequence of cell adhesion to thrombospondin-1: implications for the antiadhesive activities of thrombospondin-1. J. Cell Sci., 108, 1977–1990. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

