Abl tyrosine kinases are required for infection by Shigella flexneri
- PMID: 14532119
- PMCID: PMC213767
- DOI: 10.1093/emboj/cdg512
Abl tyrosine kinases are required for infection by Shigella flexneri
Erratum in
- EMBO J. 2003 Nov 3;22(21):5962
Abstract
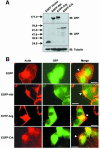
Infection by the opportunistic bacterial pathogen Shigella flexneri stimulates tyrosine phosphorylation of host cell proteins, but the kinases involved and their effects on the regulation of cell signaling pathways during bacterial entry remain largely undefined. Here, we demonstrate a requirement for the Abl family of tyrosine kinases during Shigella internalization. Family members Abl and Arg are catalytically activated upon Shigella infection, accumulate at the site of bacterial entry, and are required for efficient bacterial uptake, as internalization is blocked upon targeted deletion of these kinases or treatment with a specific pharmacological inhibitor. We identify the adapter protein Crk as a target for Abl kinases during Shigella uptake, and show that a phosphorylation-deficient Crk mutant significantly inhibits bacterial uptake. Moreover, we define a novel signaling pathway activated during Shigella entry that links Abl kinase phosphorylation of Crk to activation of the Rho family GTPases Rac and Cdc42. Together, these findings reveal a new role for the Abl kinases, and suggest a novel approach to treatment of Shigella infections through inhibition of host cell signaling pathways.
Figures

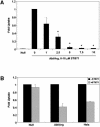
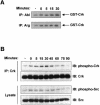
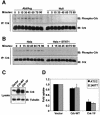
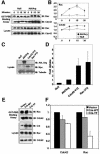
References
-
- Bagrodia S., Taylor,S.J., Jordon,K.A., Van Aelst,L. and Cerione,R.A. (1998) A novel regulator of p21-activated kinases. J. Biol. Chem., 273, 23633–23636. - PubMed
-
- Bateman J., Shu,H. and Van Vactor,D. (2000) The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron, 26, 93–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

