Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion
- PMID: 14532127
- PMCID: PMC213771
- DOI: 10.1093/emboj/cdg516
Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion
Abstract
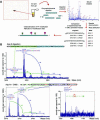
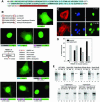
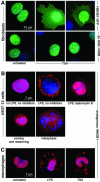
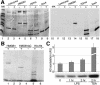
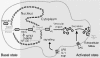
High Mobility Group 1 protein (HMGB1) is a chromatin component that, when leaked out by necrotic cells, triggers inflammation. HMGB1 can also be secreted by activated monocytes and macrophages, and functions as a late mediator of inflammation. Secretion of a nuclear protein requires a tightly controlled relocation program. We show here that in all cells HMGB1 shuttles actively between the nucleus and cytoplasm. Monocytes and macrophages acetylate HMGB1 extensively upon activation with lipopolysaccharide; moreover, forced hyperacetylation of HMGB1 in resting macrophages causes its relocalization to the cytosol. Cytosolic HMGB1 is then concentrated by default into secretory lysosomes, and secreted when monocytic cells receive an appropriate second signal.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

