The roles of initiation factor 2 and guanosine triphosphate in initiation of protein synthesis
- PMID: 14532131
- PMCID: PMC213779
- DOI: 10.1093/emboj/cdg525
The roles of initiation factor 2 and guanosine triphosphate in initiation of protein synthesis
Abstract
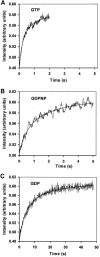
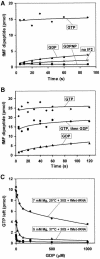
The role of IF2 from Escherichia coli was studied in vitro using a system for protein synthesis with purified components. Stopped flow experiments with light scattering show that IF2 in complex with guanosine triphosphate (GTP) or a non-cleavable GTP analogue (GDPNP), but not with guanosine diphosphate (GDP), promotes fast association of ribosomal subunits during initiation. Biochemical experiments show that IF2 promotes fast formation of the first peptide bond in the presence of GTP, but not GDPNP or GDP, and that IF2-GDPNP binds strongly to post-initiation ribosomes. We conclude that the GTP form of IF2 accelerates formation of the 70S ribosome from subunits and that GTP hydrolysis accelerates release of IF2 from the 70S ribosome. The results of a recent report, suggesting that GTP and GDP promote initiation equally fast, have been addressed. Our data, indicating that eIF5B and IF2 have similar functions, are used to rationalize the phenotypes of GTPase-deficient mutants of eIF5B and IF2.
Figures
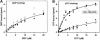



References
-
- Benne R., Naaktgeboren,N., Gubbens,J. and Voorma,H.O. (1973) Recycling of initiation factors IF-1, IF-2 and IF-3. Eur. J. Biochem., 32, 372–380. - PubMed
-
- Dahlquist K.D. and Puglisi,J.D. (2000) Interaction of translation initiation factor IF1 with the E. coli ribosomal A site. J. Mol. Biol., 299, 1–15. - PubMed
-
- Dubnoff J.S., Lockwood,A.H. and Maitra,U. (1972) Studies on the role of guanosine triphosphate in polypeptide chain initiation in Escherichia coli. J. Biol. Chem., 247, 2884–2894. - PubMed
-
- Ehrenberg M., Bilgin,N. and Kurland,C.G. (1990) Design and use of a fast and accurate in vitro translation system. In Spedding,G. (ed.), Ribosomes and Protein Synthesis: A Practical Approach. Oxford University Press, New York, NY, pp. 101–129.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

