Distinct regulators for Plk1 activation in starfish meiotic and early embryonic cycles
- PMID: 14532135
- PMCID: PMC213789
- DOI: 10.1093/emboj/cdg535
Distinct regulators for Plk1 activation in starfish meiotic and early embryonic cycles
Abstract
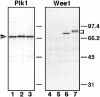
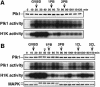
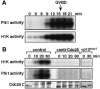
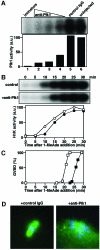
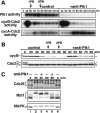
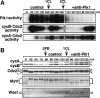
The Polo-like kinase, Plk, has multiple roles in regulating mitosis. In particular, Plk1 has been postulated to function as a trigger kinase that phosphorylates and activates Cdc25C prior to the activation of cyclin B-Cdc2 and thereby initiates its activation. However, the upstream regulation of Plk1 activation remains unclear. Here we have studied the interplay between Plk1 and Cdc2 through meiotic and early embryonic cycles in starfish. Distinct kinases, cyclin B-Cdc2, MAPK along with cyclin B- and/or cyclin A-Cdc2 and cyclin A-Cdc2, were unique upstream regulators for Plk1 activation at meiosis I, meiosis II and embryonic M-phase, respectively, indicating that Plk1 is not the trigger kinase at meiotic reinitiation. When Plk1 was required for cyclin B-Cdc2 activation, the action of Plk1 was mediated primarily through suppression of Myt1 rather than through activation of Cdc25. We propose that Plk1 can be activated by either cyclin A- or cyclin B-Cdc2, and its primary target is Myt1.
Figures



Similar articles
-
In vivo regulation of cyclin A/Cdc2 and cyclin B/Cdc2 through meiotic and early cleavage cycles in starfish.Dev Biol. 1998 May 1;197(1):39-53. doi: 10.1006/dbio.1998.8881. Dev Biol. 1998. PMID: 9578617
-
SGK phosphorylates Cdc25 and Myt1 to trigger cyclin B-Cdk1 activation at the meiotic G2/M transition.J Cell Biol. 2019 Nov 4;218(11):3597-3611. doi: 10.1083/jcb.201812122. Epub 2019 Sep 19. J Cell Biol. 2019. PMID: 31537708 Free PMC article.
-
The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase.Cell Signal. 2000 Jun;12(6):405-11. doi: 10.1016/s0898-6568(00)00080-2. Cell Signal. 2000. PMID: 11202906
-
A primer on meiotic resumption in starfish oocytes: the proposed signaling pathway triggered by maturation-inducing hormone.Mol Reprod Dev. 2011 Oct-Nov;78(10-11):704-7. doi: 10.1002/mrd.21343. Epub 2011 Jun 28. Mol Reprod Dev. 2011. PMID: 21714029 Review.
-
Cell cycle arrest and release in starfish oocytes and eggs.Semin Cell Dev Biol. 1998 Oct;9(5):549-57. doi: 10.1006/scdb.1998.0249. Semin Cell Dev Biol. 1998. PMID: 9835643 Review.
Cited by
-
Antisense morpholino targeting just upstream from a poly(A) tail junction of maternal mRNA removes the tail and inhibits translation.Nucleic Acids Res. 2012 Dec;40(22):e173. doi: 10.1093/nar/gks765. Epub 2012 Aug 16. Nucleic Acids Res. 2012. PMID: 22904086 Free PMC article.
-
Specialization of CDK1 and cyclin B paralog functions in a coenocystic mode of oogenic meiosis.Cell Cycle. 2018;17(12):1425-1444. doi: 10.1080/15384101.2018.1486167. Epub 2018 Jul 23. Cell Cycle. 2018. PMID: 29969934 Free PMC article.
-
Plk1 inhibition leads to a failure of mitotic division during the first mitotic division in pig embryos.J Assist Reprod Genet. 2017 Mar;34(3):399-407. doi: 10.1007/s10815-016-0864-4. Epub 2017 Jan 10. J Assist Reprod Genet. 2017. PMID: 28074435 Free PMC article.
-
The Plk1 inhibitor BI 2536 temporarily arrests primary cardiac fibroblasts in mitosis and generates aneuploidy in vitro.PLoS One. 2010 Sep 24;5(9):e12963. doi: 10.1371/journal.pone.0012963. PLoS One. 2010. PMID: 20886032 Free PMC article.
-
Cyclin B-Cdk1 inhibits protein phosphatase PP2A-B55 via a Greatwall kinase-independent mechanism.J Cell Biol. 2014 Mar 17;204(6):881-9. doi: 10.1083/jcb.201307160. Epub 2014 Mar 10. J Cell Biol. 2014. PMID: 24616226 Free PMC article.
References
-
- Abrieu A., Brassac,T., Galas,S., Fisher,D., Labbe,J.C. and Doree,M. (1998) The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci., 111, 1751–1757. - PubMed
-
- Brazil D.P. and Hemmings,B.A. (2001) Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci., 26, 657–664. - PubMed
-
- Coleman T.R. and Dunphy,W.G. (1994) Cdc2 regulatory factors. Curr. Opin. Cell Biol., 6, 877–882. - PubMed
-
- Devault A., Fesquet,D., Cavadore,J.C., Garrigues,A.M., Labbe,J.C., Lorca,T., Picard,A., Philippe,M. and Doree,M. (1992) Cyclin A potentiates maturation-promoting factor activation in the early Xenopus embryo via inhibition of the tyrosine kinase that phosphorylates cdc2. J. Cell Biol., 118, 1109–1120. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

