Quantifying generalization from trial-by-trial behavior of adaptive systems that learn with basis functions: theory and experiments in human motor control
- PMID: 14534237
- PMCID: PMC6740843
- DOI: 10.1523/JNEUROSCI.23-27-09032.2003
Quantifying generalization from trial-by-trial behavior of adaptive systems that learn with basis functions: theory and experiments in human motor control
Abstract
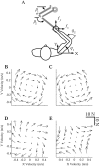
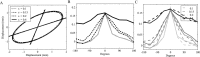
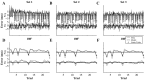
During reaching movements, the brain's internal models map desired limb motion into predicted forces. When the forces in the task change, these models adapt. Adaptation is guided by generalization: errors in one movement influence prediction in other types of movement. If the mapping is accomplished with population coding, combining basis elements that encode different regions of movement space, then generalization can reveal the encoding of the basis elements. We present a theory that relates encoding to generalization using trial-by-trial changes in behavior during adaptation. We consider adaptation during reaching movements in various velocity-dependent force fields and quantify how errors generalize across direction. We find that the measurement of error is critical to the theory. A typical assumption in motor control is that error is the difference between a current trajectory and a desired trajectory (DJ) that does not change during adaptation. Under this assumption, in all force fields that we examined, including one in which force randomly changes from trial to trial, we found a bimodal generalization pattern, perhaps reflecting basis elements that encode direction bimodally. If the DJ was allowed to vary, bimodality was reduced or eliminated, but the generalization function accounted for nearly twice as much variance. We suggest, therefore, that basis elements representing the internal model of dynamics are sensitive to limb velocity with bimodal tuning; however, it is also possible that during adaptation the error metric itself adapts, which affects the implied shape of the basis elements.
Figures







Similar articles
-
Error generalization as a function of velocity and duration: human reaching movements.Exp Brain Res. 2008 Mar;186(1):23-37. doi: 10.1007/s00221-007-1202-y. Epub 2007 Nov 20. Exp Brain Res. 2008. PMID: 18030456
-
Adaptation and generalization in acceleration-dependent force fields.Exp Brain Res. 2006 Mar;169(4):496-506. doi: 10.1007/s00221-005-0163-2. Epub 2005 Nov 16. Exp Brain Res. 2006. PMID: 16292640 Free PMC article.
-
A gain-field encoding of limb position and velocity in the internal model of arm dynamics.PLoS Biol. 2003 Nov;1(2):E25. doi: 10.1371/journal.pbio.0000025. Epub 2003 Nov 17. PLoS Biol. 2003. PMID: 14624237 Free PMC article.
-
Internal models of limb dynamics and the encoding of limb state.J Neural Eng. 2005 Sep;2(3):S266-78. doi: 10.1088/1741-2560/2/3/S09. Epub 2005 Aug 31. J Neural Eng. 2005. PMID: 16135889 Free PMC article. Review.
-
Learning arm kinematics and dynamics.Annu Rev Neurosci. 1989;12:157-83. doi: 10.1146/annurev.ne.12.030189.001105. Annu Rev Neurosci. 1989. PMID: 2648948 Review.
Cited by
-
An analytical method reduces noise bias in motor adaptation analysis.Sci Rep. 2021 Apr 29;11(1):9245. doi: 10.1038/s41598-021-88688-5. Sci Rep. 2021. PMID: 33927273 Free PMC article.
-
Behavioural and neural basis of anomalous motor learning in children with autism.Brain. 2015 Mar;138(Pt 3):784-97. doi: 10.1093/brain/awu394. Epub 2015 Jan 20. Brain. 2015. PMID: 25609685 Free PMC article.
-
Flexible Control of Safety Margins for Action Based on Environmental Variability.J Neurosci. 2015 Jun 17;35(24):9106-21. doi: 10.1523/JNEUROSCI.1883-14.2015. J Neurosci. 2015. PMID: 26085634 Free PMC article. Clinical Trial.
-
Neuromuscular-skeletal constraints on the acquisition of skill in a discrete torque production task.Exp Brain Res. 2006 Nov;175(3):400-10. doi: 10.1007/s00221-006-0547-y. Epub 2006 Sep 28. Exp Brain Res. 2006. PMID: 17006690
-
Effects of human cerebellar thalamus disruption on adaptive control of reaching.Cereb Cortex. 2006 Oct;16(10):1462-73. doi: 10.1093/cercor/bhj087. Epub 2005 Dec 15. Cereb Cortex. 2006. PMID: 16357337 Free PMC article. Clinical Trial.
References
-
- Ahissar M, Hochstein S ( 1997) Task difficulty and the specificity of perceptual learning. Nature 387: 401-406. - PubMed
-
- Bosco G, Rankin A, Poppele R ( 1996) Representation of passive hindlimb postures in cat spinocerebellar activity. J Neurophysiol 76: 715-726. - PubMed
-
- Brashers-Krug T, Shadmehr R, Bizzi E ( 1996) Consolidation in human motor memory. Nature 382: 252-255. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
