Mechanisms of mitochondria-neurofilament interactions
- PMID: 14534238
- PMCID: PMC6740819
- DOI: 10.1523/JNEUROSCI.23-27-09046.2003
Mechanisms of mitochondria-neurofilament interactions
Abstract
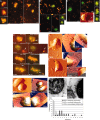
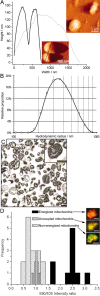
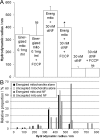
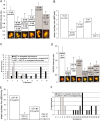
Mitochondria are localized to regions of the cell where ATP consumption is high and are dispersed according to changes in local energy needs. In addition to motion directed by molecular motors, mitochondrial distribution in neuronal cells appears to depend on the docking of mitochondria to microtubules and neurofilaments. We examined interactions between mitochondria and neurofilaments using fluorescence microscopy, dynamic light scattering, atomic force microscopy, and sedimentation assays. Mitochondria-neurofilament interactions depend on mitochondrial membrane potential, as revealed by staining with a membrane potential sensitive dye (JC-1) in the presence of substrates/ADP or uncouplers (valinomycin/carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone) and are affected by the phosphorylation status of neurofilaments and neurofilament sidearms. Antibodies against the neurofilament heavy subunit disrupt binding between mitochondria and neurofilaments, and isolated neurofilament sidearms alone interact with mitochondria, suggesting that they mediate the interactions between the two structures. These data suggest that specific and regulated mitochondrial-neurofilament interactions occur in situ and may contribute to the dynamic distribution of these organelles within the cytoplasm of neurons.
Figures



References
-
- Almahbobi G, Williams LJ, Han XG, Hall PF ( 1993) Binding of lipid droplets and mitochondria to intermediate filaments in rat Leydig cells. J Reprod Fertil 98: 209-217. - PubMed
-
- Aranda-Espinoza H, Carl P, Leterrier JF, Janmey P, Discher DE ( 2002) Domain unfolding in neurofilament sidearms: effects of phosphorylation and ATP. FEBS Lett 531: 397-401. - PubMed
-
- Aw TY, Jones DP ( 1988) Microzonation of ATP and pH in the aqueous cytoplasm of mammalian cells. In: Microcompartmentation, pp 191-208. Boca Raton, FL: CRC.
-
- Benshalom G, Reese TS ( 1985) Ultrastructural observations on the cytoarchitecture of axons processed by rapid-freezing and freeze-substitution. J Neurocytol 14: 943-960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
