Dorsal horn neurons firing at high frequency, but not primary afferents, release opioid peptides that produce micro-opioid receptor internalization in the rat spinal cord
- PMID: 14534251
- PMCID: PMC2561240
- DOI: 10.1523/JNEUROSCI.23-27-09171.2003
Dorsal horn neurons firing at high frequency, but not primary afferents, release opioid peptides that produce micro-opioid receptor internalization in the rat spinal cord
Abstract
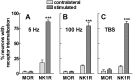
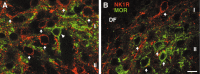
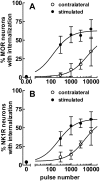
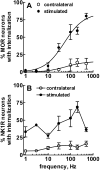
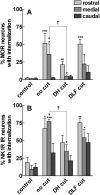
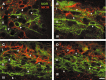
To determine what neural pathways trigger opioid release in the dorsal horn, we stimulated the dorsal root, the dorsal horn, or the dorsolateral funiculus (DLF) in spinal cord slices while superfusing them with peptidase inhibitors to prevent opioid degradation. Internalization of mu-opioid receptors (MOR) and neurokinin 1 receptors (NK1R) was measured to assess opioid and neurokinin release, respectively. Dorsal root stimulation at low, high, or mixed frequencies produced abundant NK1R internalization but no MOR internalization, indicating that primary afferents do not release opioids. Moreover, capsaicin and NMDA also failed to produce MOR internalization. In contrast, dorsal horn stimulation elicited MOR internalization that increased with the frequency, being negligible at <10 Hz and maximal at 500 Hz. The internalization was abolished by the MOR antagonist D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP), in the presence of low Ca2+ and by the Na+ channel blocker lidocaine, confirming that it was caused by opioid release and neuronal firing. DLF stimulation in "oblique" slices (encompassing the DLF and the dorsal horn of T11-L4) produced MOR internalization, but only in areas near the stimulation site. Moreover, cutting oblique slices across the dorsal horn (but not across the DLF) eliminated MOR internalization in areas distal to the cut, indicating that it was produced by signals traveling in the dorsal horn and not via the DLF. These findings demonstrate that some dorsal horn neurons release opioids when they fire at high frequencies, perhaps by integrating signals from the rostral ventromedial medulla, primary afferents, and other areas of the spinal cord.
Figures




Similar articles
-
Peptidases prevent mu-opioid receptor internalization in dorsal horn neurons by endogenously released opioids.J Neurosci. 2003 Mar 1;23(5):1847-58. doi: 10.1523/JNEUROSCI.23-05-01847.2003. J Neurosci. 2003. PMID: 12629189 Free PMC article.
-
Endogenous opioid peptides acting at mu-opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons.J Neurophysiol. 1998 Feb;79(2):677-87. doi: 10.1152/jn.1998.79.2.677. J Neurophysiol. 1998. PMID: 9463431
-
Noxious mechanical stimulation evokes the segmental release of opioid peptides that induce mu-opioid receptor internalization in the presence of peptidase inhibitors.Brain Res. 2008 Mar 4;1197:85-93. doi: 10.1016/j.brainres.2007.12.040. Epub 2008 Jan 3. Brain Res. 2008. PMID: 18207137 Free PMC article.
-
GABA(A) receptor facilitation of neurokinin release from primary afferent terminals in the rat spinal cord.Neuroscience. 2005;130(4):1013-27. doi: 10.1016/j.neuroscience.2004.10.019. Neuroscience. 2005. PMID: 15652997
-
Neurokinin release produced by capsaicin acting on the central terminals and axons of primary afferents: relationship with N-methyl-D-aspartate and GABA(B) receptors.Neuroscience. 2003;121(3):667-80. doi: 10.1016/s0306-4522(03)00501-3. Neuroscience. 2003. PMID: 14568027
Cited by
-
Neuropeptide Y release in the rat spinal cord measured with Y1 receptor internalization is increased after nerve injury.Neuropharmacology. 2019 Nov 1;158:107732. doi: 10.1016/j.neuropharm.2019.107732. Epub 2019 Aug 2. Neuropharmacology. 2019. PMID: 31377198 Free PMC article.
-
Inhibition of opioid release in the rat spinal cord by serotonin 5-HT(1A) receptors.Brain Res. 2007 Jul 16;1158:57-62. doi: 10.1016/j.brainres.2007.05.006. Epub 2007 May 8. Brain Res. 2007. PMID: 17555728 Free PMC article.
-
Substance P induces the reversible formation of varicosities in the dendrites of rat brainstem neurons.Brain Res. 2011 Jan 19;1369:36-45. doi: 10.1016/j.brainres.2010.10.091. Epub 2010 Oct 31. Brain Res. 2011. PMID: 21044613 Free PMC article.
-
N-methyl-D-aspartate receptors and large conductance calcium-sensitive potassium channels inhibit the release of opioid peptides that induce mu-opioid receptor internalization in the rat spinal cord.Neuroscience. 2005;136(2):549-62. doi: 10.1016/j.neuroscience.2005.08.032. Epub 2005 Oct 3. Neuroscience. 2005. PMID: 16203108 Free PMC article.
-
Endogenous analgesia, dependence, and latent pain sensitization.Curr Top Behav Neurosci. 2014;20:283-325. doi: 10.1007/7854_2014_351. Curr Top Behav Neurosci. 2014. PMID: 25227929 Free PMC article.
References
-
- Abbadie C, Pasternak GW, Aicher SA ( 2001) Presynaptic localization of the carboxy-terminus epitopes of the mu opioid receptor splice variants MOR-1C and MOR-1D in the superficial laminae of the rat spinal cord. Neuroscience 106: 833-842. - PubMed
-
- Afrah AW, Stiller CO, Olgart L, Brodin E, Gustafsson H ( 2001) Involvement of spinal N-methyl-d-aspartate receptors in capsaicin-induced in vivo release of substance P in the rat dorsal horn. Neurosci Lett 316: 83-86. - PubMed
-
- Aimone LD, Yaksh TL ( 1989) Opioid modulation of capsaicin-evoked release of substance P from rat spinal cord in vivo. Peptides 10: 1127-1131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
