A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles
- PMID: 14534255
- PMCID: PMC6740823
- DOI: 10.1523/JNEUROSCI.23-27-09208.2003
A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles
Abstract
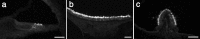
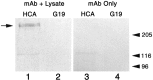
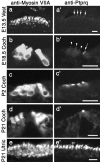
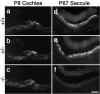
A screen for protein tyrosine phosphatases (PTPs) expressed in the chick inner ear yielded a high proportion of clones encoding an avian ortholog of protein tyrosine phosphatase receptor Q (Ptprq), a receptor-like PTP. Ptprq was first identified as a transcript upregulated in rat kidney in response to glomerular nephritis and has recently been shown to be active against inositol phospholipids. An antibody to the intracellular domain of Ptprq, anti-Ptprq, stains hair bundles in mice and chicks. In the chick ear, the distribution of Ptprq is almost identical to that of the 275 kDa hair-cell antigen (HCA), a component of hair-bundle shaft connectors recognized by a monoclonal antibody (mAb) that stains inner-ear hair bundles and kidney glomeruli. Furthermore, anti-Ptprq immunoblots a 275 kDa polypeptide immunoprecipitated by the anti-HCA mAb from the avian inner ear, indicating that the HCA and Ptprq are likely to be the same molecule. In two transgenic mouse strains with different mutations in Ptprq, anti-Ptprq immunoreactivity cannot be detected in the ear. Shaft connectors are absent from mutant vestibular hair bundles, but the stereocilia forming the hair bundle are not splayed, indicating that shaft connectors are not necessary to hold the stereocilia together; however, the mice show rapid postnatal deterioration in cochlear hair-bundle structure, associated with smaller than normal transducer currents with otherwise normal adaptation properties, a progressive loss of basal-coil cochlear hair cells, and deafness. These results reveal that Ptprq is required for formation of the shaft connectors of the hair bundle, the normal maturation of cochlear hair bundles, and the long-term survival of high-frequency auditory hair cells.
Figures









References
-
- Alagraman KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP ( 2001) The mouse Ames waltzer hearing-loss mutant is caused by a mutation of Pdch15, a novel protocadherin gene. Nat Genet 27: 99-102. - PubMed
-
- Altschul F, Gish W, Miller W, Myers EW, Lipman DJ ( 1990) Basic local alignment research tool. J Mol Biol 215: 403-410. - PubMed
-
- Anniko M ( 1983) Cytodifferentiation of cochlear hair cells. Am J Otolaryngol 4: 375-388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
