Cyclothiazide potently inhibits gamma-aminobutyric acid type A receptors in addition to enhancing glutamate responses
- PMID: 14534329
- PMCID: PMC240738
- DOI: 10.1073/pnas.2133370100
Cyclothiazide potently inhibits gamma-aminobutyric acid type A receptors in addition to enhancing glutamate responses
Abstract
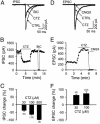
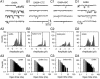
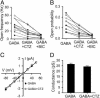
Ionotropic glutamate and gamma-aminobutyric acid type A (GABAA) receptors mediate critical excitatory and inhibitory actions in the brain. Cyclothiazide (CTZ) is well known for its effect of enhancing glutamatergic transmission and is widely used as a blocker for alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptor desensitization. Here, we report that in addition to its action on AMPA receptors, CTZ also exerts a powerful but opposite effect on GABAA receptors. We found that CTZ reversibly inhibited both evoked and spontaneous inhibitory postsynaptic currents, as well as GABA application-induced membrane currents, in a dose-dependent manner. Single-channel analyses revealed further that CTZ greatly reduced the open probability of GABAA receptor channels. These results demonstrate that CTZ interacts with both glutamate and GABAA receptors and shifts the excitation-inhibition balance in the brain by two independent mechanisms. Understanding the molecular mechanism of this double-faceted drug-receptor interaction may help in designing new therapies for neurological diseases.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

