Targeted chemical disruption of clathrin function in living cells
- PMID: 14551251
- PMCID: PMC266763
- DOI: 10.1091/mbc.e03-04-0230
Targeted chemical disruption of clathrin function in living cells
Abstract
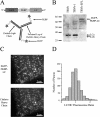
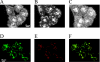
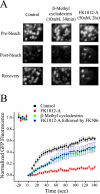
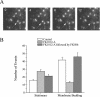
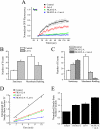
The accurate assignment of molecular roles in membrane traffic is frequently complicated by the lack of specific inhibitors that can work on rapid time scales. Such inhibition schemes would potentially avoid the complications arising from either compensatory gene expression or the complex downstream consequences of inhibition of an important protein over long periods (>12 h). Here, we developed a novel chemical tool to disrupt clathrin function in living cells. We engineered a cross-linkable form of clathrin by using an FK506-binding protein 12 (FKBP)-clathrin fusion protein that is specifically oligomerized upon addition of the cell-permeant cross-linker FK1012-A. This approach interrupts the normal assembly-disassembly cycle of clathrin lattices and results in a specific, rapid, and reversible approximately 70% inhibition of clathrin function. This approach should be applicable to a number of proteins that must go through an assembly-disassembly cycle for normal function.
Figures


References
-
- Doxsey, S.J., Brodsky, F.M., Blank, G.S., and Helenius, A. (1987). Inhibition of endocytosis by anti-clathrin antibodies. Cell 50, 453-463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

