Diacylglycerol kinase-zeta localization in skeletal muscle is regulated by phosphorylation and interaction with syntrophins
- PMID: 14551255
- PMCID: PMC266768
- DOI: 10.1091/mbc.e03-03-0190
Diacylglycerol kinase-zeta localization in skeletal muscle is regulated by phosphorylation and interaction with syntrophins
Abstract
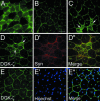
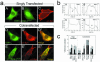
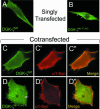
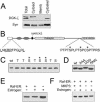
Syntrophins are scaffolding proteins that link signaling molecules to dystrophin and the cytoskeleton. We previously reported that syntrophins interact with diacylglycerol kinase-zeta (DGK-zeta), which phosphorylates diacylglycerol to yield phosphatidic acid. Here, we show syntrophins and DGK-zeta form a complex in skeletal muscle whose translocation from the cytosol to the plasma membrane is regulated by protein kinase C-dependent phosphorylation of the DGK-zeta MARCKS domain. DGK-zeta mutants that do not bind syntrophins were mislocalized, and an activated mutant of this sort induced atypical changes in the actin cytoskeleton, indicating syntrophins are important for localizing DGK-zeta and regulating its activity. Consistent with a role in actin organization, DGK-zeta and syntrophins were colocalized with filamentous (F)-actin and Rac in lamellipodia and ruffles. Moreover, extracellular signal-related kinase-dependent phosphorylation of DGK-zeta regulated its association with the cytoskeleton. In adult muscle, DGK-zeta was colocalized with syntrophins on the sarcolemma and was concentrated at neuromuscular junctions (NMJs), whereas in type IIB fibers it was found exclusively at NMJs. DGK-zeta was reduced at the sarcolemma of dystrophin-deficient mdx mouse myofibers but was specifically retained at NMJs, indicating that dystrophin is important for the sarcolemmal but not synaptic localization of DGK-zeta. Together, our findings suggest syntrophins localize DGK-zeta signaling complexes at specialized domains of muscle cells, which may be critical for the proper control of lipid-signaling pathways regulating actin organization. In dystrophic muscle, mislocalized DGK-zeta may cause abnormal cytoskeletal changes that contribute to disease pathogenesis.
Figures






References
-
- Adams, M.E., Butler, M.H., Dwyer, T.M., Peters, M.F., Murnane, A.A., and Froehner, S.C. (1993). Two forms of mouse syntrophin, a 58 kD dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron 11, 531-540. - PubMed
-
- Adams, M.E., Dwyer, T.M., Dowler, L.L., White, R.A., and Froehner, S.C. (1995). Mouse alpha 1- and beta 2-syntrophin gene structure, chromosome localization, and homology with a discs large domain. J. Biol. Chem. 270, 25859-25865. - PubMed
-
- Ahn, A.H., Freener, C.A., Gussoni, E., Yoshida, M., Ozawa, E., and Kunkel, L.M. (1996). The three human syntrophin genes are expressed in diverse tissues, have distinct chromosomal locations, and each bind to dystrophin and its relatives. J. Biol. Chem. 271, 2724-2730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

