Signal transduction crosstalk in the endocrine system: pancreatic beta-cells and the glucose competence concept
- PMID: 1455507
- PMCID: PMC2925193
- DOI: 10.1016/0968-0004(92)90006-u
Signal transduction crosstalk in the endocrine system: pancreatic beta-cells and the glucose competence concept
Abstract
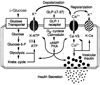
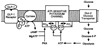
Crosstalk between intracellular signalling systems is recognized as the principal means by which a cell orchestrates coordinate responses to stimulation by neurotransmitters, hormones or growth factors. The functional consequences of crosstalk are evident at multiple levels within a given signalling cascade, including the regulation of receptor-ligand interactions, guanine nucleotide-binding proteins, enzyme activities, ion channel function and gene expression. Here we focus on the pancreatic beta-cells of the islets of Langerhans to illustrate the important role crosstalk plays in the regulation of glucose-induced insulin secretion. Recent studies indicating a synergistic interaction in beta-cells between the glucose-regulated ATP-dependent signalling system and the hormonally regulated cAMP-dependent signalling system are emphasized. This interaction gives beta-cells the ability to match the ambient concentration of glucose to an appropriate insulin secretory response, a process we refer to as the induction of glucose competence. The glucose competence concept may provide new insights into the etiology and treatment of non-insulin-dependent diabetes mellitus (Type II diabetes).
Figures


References
-
- Wollheim CB, Sharp GWG. Physiol. Rev. 1981;61:914–973. - PubMed
-
- Zawalich WS, Rasmussen H. Mol. Cell. Endocrinol. 1990;70:119–137. - PubMed
-
- Matschinsky FM. Diabetes. 1990;39:647–652. - PubMed
-
- Bell GI, et al. Diabetes Care. 1990;13:198–208. - PubMed
-
- Thorens B, Charron MJ, Lodish HF. Diabetes Care. 1990;13:209–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

