Regulation of ENA1 Na(+)-ATPase gene expression by the Ppz1 protein phosphatase is mediated by the calcineurin pathway
- PMID: 14555476
- PMCID: PMC219373
- DOI: 10.1128/EC.2.5.937-948.2003
Regulation of ENA1 Na(+)-ATPase gene expression by the Ppz1 protein phosphatase is mediated by the calcineurin pathway
Abstract
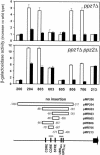
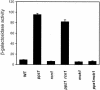
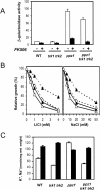
Saccharomyces cerevisiae strains lacking the Ppz1 protein phosphatase are salt tolerant and display increased expression of the ENA1 Na(+)-ATPase gene, a major determinant for sodium extrusion, while cells devoid of the similar Ppz2 protein do not show these phenotypes. However, a ppz1 ppz2 mutant displays higher levels of ENA1 expression than the ppz1 strain. We show here that the increased activity of the ENA1 promoter in a ppz1 ppz2 mutant maps to two regions: one region located at -751 to -667, containing a calcineurin-dependent response element (CDRE), and one downstream region (-573 to -490) whose activity responds to intracellular alkalinization. In contrast, the increased ENA1 expression in a ppz1 mutant is mediated solely by an intact calcineurin/Crz1 signaling pathway, on the basis that (i) this effect maps to a single region that contains the CDRE and (ii) it is blocked by the calcineurin inhibitor FK506, as well as by deletion of the CNB1 or CRZ1 gene. The calcineurin dependence of the increased ENA1 expression of a ppz1 mutant would suggest that Ppz1 could negatively regulate calcineurin activity. In agreement with this notion, a ppz1 strain is calcium sensitive, and this mutation does not result in a decrease in the calcium hypertolerance of a cnb1 mutant. It has been shown that ENA1 can be induced by alkalinization of the medium and that a ppz1 ppz2 strain has a higher intracellular pH. However, we present several lines of evidence that show that the gene expression profile of a ppz1 mutant does not involve an alkalinization effect. In conclusion, we have identified a novel role for calcineurin, but not alkalinization, in the control of ENA1 expression in ppz1 mutants.
Figures
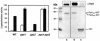
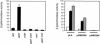
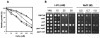


References
-
- Adams, A., D. E. Gottschlings, C. A. Kaiser, and T. Stearns. 1998. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
-
- Alepuz, P. M., K. W. Cunningham, and F. Estruch. 1997. Glucose repression affects ion homeostasis in yeast through the regulation of the stress-activated ENA1 gene. Mol. Microbiol. 26:91-98. - PubMed
-
- Ariño, J., F. Posas, and J. Clotet. 1998. The search for the biological function of novel yeast Ser/Thr phosphatases. Methods Mol. Biol. 93:305-313. - PubMed
-
- Berben, G., J. Dumont, V. Gilliquet, P. A. Bolle, and F. Hilger. 1991. The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast 7:475-477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

