Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery
- PMID: 14555724
- PMCID: PMC2343487
- DOI: 10.1113/jphysiol.2003.051896
Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery
Abstract
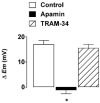
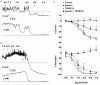
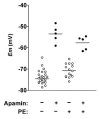
Activation of both small-conductance (SKCa) and intermediate-conductance (IKCa) Ca2+-activated K+ channels in endothelial cells leads to vascular smooth muscle hyperpolarization and relaxation in rat mesenteric arteries. The contribution that each endothelial K+ channel type makes to the smooth muscle hyperpolarization is unknown. In the presence of a nitric oxide (NO) synthase inhibitor, ACh evoked endothelium and concentration-dependent smooth muscle hyperpolarization, increasing the resting potential (approx. -53 mV) by around 20 mV at 3 microM. Similar hyperpolarization was evoked with cyclopiazonic acid (10 microM, an inhibitor of sarcoplasmic endoplasmic reticulum calcium ATPase (SERCA)) while 1-EBIO (300 microM, an IKCa activator) only increased the potential by a few millivolts. Hyperpolarization in response to either ACh or CPA was abolished with apamin (50 nM, an SKCa blocker) but was unaltered by 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (1 microM TRAM-34, an IKCa blocker). During depolarization and contraction in response to phenylephrine (PE), ACh still increased the membrane potential to around -70 mV, but with apamin present the membrane potential only increased just beyond the original resting potential (circa -58 mV). TRAM-34 alone did not affect hyperpolarization to ACh but, in combination with apamin, ACh-evoked hyperpolarization was completely abolished. These data suggest that true endothelium-dependent hyperpolarization of smooth muscle cells in response to ACh is attributable to SKCa channels, whereas IKCa channels play an important role during the ACh-mediated repolarization phase only observed following depolarization.
Figures
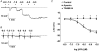
References
-
- Barriere E, Tazi KA, Pessione F, Heller J, Poirel O, Lebrec D, Moreau R. Role of small-conductance Ca2+-dependent K+ channels in in vitro nitric oxide-mediated aortic hyporeactivity to alpha-adrenergic vasoconstriction in rats with cirrhosis. J Hepatol. 2001;35:350–357. - PubMed
-
- Burdyga T, Shmygol A, Eisner DA, Wray S. A new technique for simultaneous and in situ measurements of Ca2+ signals in arteriolar smooth muscle and endothelial cells. Cell Calcium. 2003;34:27–33. - PubMed
-
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

