The mechanism of the force response to stretch in human skinned muscle fibres with different myosin isoforms
- PMID: 14555725
- PMCID: PMC1664769
- DOI: 10.1113/jphysiol.2003.051748
The mechanism of the force response to stretch in human skinned muscle fibres with different myosin isoforms
Erratum in
- J Physiol. 2004 Mar 16;555(Pt 3):851
Abstract
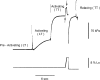
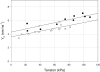
Force enhancement during lengthening of an active muscle, a condition that normally occurs during locomotion in vivo, is attributed to recruitment of myosin heads that exhibit fast attachment to and detachment from actin in a cycle that does not imply ATP splitting. We investigated the kinetic and mechanical features of this cycle in Ca(2+) activated single skinned fibres from human skeletal muscles containing different myosin heavy chain (MHC) isoforms, identified with single-fibre gel electrophoresis. Fibres were activated by using a new set-up that allows development of most of the tension following a temperature jump from 0-1 degrees C to the test temperature (approximately 12 degrees C). In this way we could prevent the development of sarcomere non-uniformity and record sarcomere length changes with a striation follower in any phase of the mechanical protocol. We found that: (i) fibres with fast MHC isoforms develop 40-70% larger isometric forces than those with slow isoforms, as a result of both a larger fraction of force-generating myosin heads and a higher force per head; (ii) in both slow and fast fibres, force enhancement by stretch is due to recruitment of myosin head attachments, without increase in strain per head above the value generated by the isometric heads; and (iii) the extent of recruitment is larger in slow fibres than in fast fibres, so that the steady force and power output elicited by lengthening become similar, indicating that mechanical and kinetic properties of the actin-myosin interactions under stretch become independent of the MHC isoform.
Figures








Similar articles
-
Mechanical parameters of the molecular motor myosin II determined in permeabilised fibres from slow and fast skeletal muscles of the rabbit.J Physiol. 2018 Apr 1;596(7):1243-1257. doi: 10.1113/JP275404. Epub 2018 Jan 17. J Physiol. 2018. PMID: 29148051 Free PMC article.
-
Dependence of cross-bridge kinetics on myosin light chain isoforms in rabbit and rat skeletal muscle fibres.J Physiol. 2006 Feb 15;571(Pt 1):231-42. doi: 10.1113/jphysiol.2005.099770. Epub 2005 Dec 15. J Physiol. 2006. PMID: 16357018 Free PMC article.
-
Chemo-mechanical energy transduction in relation to myosin isoform composition in skeletal muscle fibres of the rat.J Physiol. 1997 Jul 15;502 ( Pt 2)(Pt 2):449-60. doi: 10.1111/j.1469-7793.1997.449bk.x. J Physiol. 1997. PMID: 9263923 Free PMC article.
-
Muscle mechanics: adaptations with exercise-training.Exerc Sport Sci Rev. 1996;24:427-73. Exerc Sport Sci Rev. 1996. PMID: 8744258 Review.
-
Smooth, slow and smart muscle motors.J Muscle Res Cell Motil. 2003;24(2-3):165-73. doi: 10.1023/a:1026001513928. J Muscle Res Cell Motil. 2003. PMID: 14609028 Review.
Cited by
-
Single skeletal muscle fiber behavior after a quick stretch in young and older men: a possible explanation of the relative preservation of eccentric force in old age.Pflugers Arch. 2006 Jul;452(4):464-70. doi: 10.1007/s00424-006-0065-6. Epub 2006 Apr 19. Pflugers Arch. 2006. PMID: 16622703 Clinical Trial.
-
Stiffness and fraction of Myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas.Biophys J. 2007 Apr 1;92(7):2476-90. doi: 10.1529/biophysj.106.099549. Epub 2007 Jan 19. Biophys J. 2007. PMID: 17237201 Free PMC article.
-
Mechanism of force enhancement during and after lengthening of active muscle: a temperature dependence study.J Muscle Res Cell Motil. 2012 Oct;33(5):313-25. doi: 10.1007/s10974-012-9307-8. Epub 2012 Jun 16. J Muscle Res Cell Motil. 2012. PMID: 22706970
-
Force enhancement in the human vastus lateralis is muscle-length-dependent following stretch but not during stretch.Eur J Appl Physiol. 2020 Dec;120(12):2597-2610. doi: 10.1007/s00421-020-04488-1. Epub 2020 Sep 5. Eur J Appl Physiol. 2020. PMID: 32892321 Free PMC article.
-
Eccentric muscle contractions: from single muscle fibre to whole muscle mechanics.Pflugers Arch. 2023 Apr;475(4):421-435. doi: 10.1007/s00424-023-02794-z. Epub 2023 Feb 15. Pflugers Arch. 2023. PMID: 36790515 Free PMC article. Review.
References
-
- Blangé T, Stienen GJM, Treijtel BW. Active stiffness in frog skinned muscle fibres at different Ca concentrations. J Physiol. 1985;366:65P.
-
- Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Pflugers Arch. 2001;443:6–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

