Interaction of calmodulin, a sorting nexin and kinase-associated protein phosphatase with the Brassica oleracea S locus receptor kinase
- PMID: 14555783
- PMCID: PMC219065
- DOI: 10.1104/pp.103.023846
Interaction of calmodulin, a sorting nexin and kinase-associated protein phosphatase with the Brassica oleracea S locus receptor kinase
Abstract
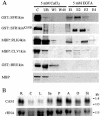
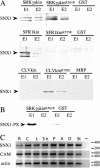
Recognition of self-pollen during the self-incompatibility response in Brassica oleracea is mediated by the binding of a secreted peptide (the S locus cysteine-rich protein) to the S locus receptor kinase (SRK), a member of the plant receptor kinase (PRK) superfamily. Here, we describe the characterization of three proteins that interact with the cytosolic kinase domain of SRK. A B. oleracea homolog of Arabidopsis kinase-associated protein phosphatase was shown to interact with and dephosphorylate SRK and was itself phosphorylated by SRK. Yeast (Saccharomyces cerevisiae) two-hybrid screens identified two additional interactors, calmodulin and a sorting nexin, both of which have been implicated in receptor kinase down-regulation in animals. A calmodulin-binding site was identified in sub-domain VIa of the SRK kinase domain. The binding site is conserved and functional in several other members of the PRK family. The sorting nexin also interacted with diverse members of the PRK family, suggesting that all three of the interacting proteins described here may play a general role in signal transduction by this family of proteins.
Figures




Similar articles
-
Two members of the thioredoxin-h family interact with the kinase domain of a Brassica S locus receptor kinase.Plant Cell. 1996 Sep;8(9):1641-50. doi: 10.1105/tpc.8.9.1641. Plant Cell. 1996. PMID: 8837514 Free PMC article.
-
Identification and characterization of BoPUB3: a novel interaction protein with S-locus receptor kinase in Brassica oleracea L.Acta Biochim Biophys Sin (Shanghai). 2019 Jul 10;51(7):723-733. doi: 10.1093/abbs/gmz057. Acta Biochim Biophys Sin (Shanghai). 2019. PMID: 31168565
-
Structural modules for receptor dimerization in the S-locus receptor kinase extracellular domain.Proc Natl Acad Sci U S A. 2007 Jul 17;104(29):12211-6. doi: 10.1073/pnas.0705186104. Epub 2007 Jul 3. Proc Natl Acad Sci U S A. 2007. PMID: 17609367 Free PMC article.
-
Just how complex is the Brassica S-receptor complex?J Exp Bot. 2003 Jan;54(380):157-68. doi: 10.1093/jxb/erg033. J Exp Bot. 2003. PMID: 12456766 Review.
-
S-locus receptor kinase signalling.Biochem Soc Trans. 2014 Apr;42(2):313-9. doi: 10.1042/BST20130222. Biochem Soc Trans. 2014. PMID: 24646237 Review.
Cited by
-
Commonalities and differences between Brassica and Arabidopsis self-incompatibility.Hortic Res. 2014 Oct 29;1:14054. doi: 10.1038/hortres.2014.54. eCollection 2014. Hortic Res. 2014. PMID: 26504553 Free PMC article. Review.
-
Identification of the phosphorylation targets of symbiotic receptor-like kinases using a high-throughput multiplexed assay for kinase specificity.Plant J. 2017 Jun;90(6):1196-1207. doi: 10.1111/tpj.13529. Epub 2017 Apr 29. Plant J. 2017. PMID: 28267253 Free PMC article.
-
Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting.Proc Natl Acad Sci U S A. 2008 Nov 18;105(46):17812-7. doi: 10.1073/pnas.0808073105. Epub 2008 Nov 12. Proc Natl Acad Sci U S A. 2008. PMID: 19004783 Free PMC article.
-
Mechanisms and concepts paving the way towards a complete transport cycle of plant vacuolar sorting receptors.Plant Cell. 2012 May;24(5):1714-32. doi: 10.1105/tpc.112.095679. Epub 2012 May 8. Plant Cell. 2012. PMID: 22570446 Free PMC article. Review.
-
Peroxisome extensions deliver the Arabidopsis SDP1 lipase to oil bodies.Proc Natl Acad Sci U S A. 2015 Mar 31;112(13):4158-63. doi: 10.1073/pnas.1403322112. Epub 2015 Mar 16. Proc Natl Acad Sci U S A. 2015. PMID: 25775518 Free PMC article.
References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 - PubMed
-
- Anonymous (2001) Yeast Protocols Hand Book. CLONTECH Laboratories Inc., Palo Alto, CA
-
- Braun DM, Stone JM, Walker JC (1997) Interaction of the maize and Arabidopsis kinase interaction domain with a subset of receptor-like protein kinases: implications for transmembrane signaling in plants. Plant J 12: 83–95 - PubMed
-
- Cabrillac D, Cock JM, Dumas C, Gaude T (2001) The S-locus receptor kinase is inhibited by thioredoxins and activated by pollen coat proteins. Nature 410: 220–223 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

