A role for Yip1p in COPII vesicle biogenesis
- PMID: 14557247
- PMCID: PMC2173432
- DOI: 10.1083/jcb.200306118
A role for Yip1p in COPII vesicle biogenesis
Abstract
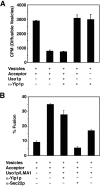
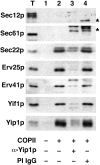
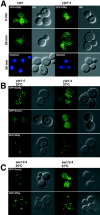
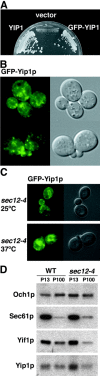
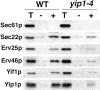
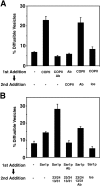
Yeast Ypt1p-interacting protein (Yip1p) belongs to a conserved family of transmembrane proteins that interact with Rab GTPases. We encountered Yip1p as a constituent of ER-derived transport vesicles, leading us to hypothesize a direct role for this protein in transport through the early secretory pathway. Using a cell-free assay that recapitulates protein transport from the ER to the Golgi complex, we find that affinity-purified antibodies directed against the hydrophilic amino terminus of Yip1p potently inhibit transport. Surprisingly, inhibition is specific to the COPII-dependent budding stage. In support of this in vitro observation, strains bearing the temperature-sensitive yip1-4 allele accumulate ER membranes at a nonpermissive temperature, with no apparent accumulation of vesicle intermediates. Genetic interaction analyses of the yip1-4 mutation corroborate a function in ER budding. Finally, ordering experiments show that preincubation of ER membranes with COPII proteins decreases sensitivity to anti-Yip1p antibodies, indicating an early requirement for Yip1p in vesicle formation. We propose that Yip1p has a previously unappreciated role in COPII vesicle biogenesis.
Figures




Similar articles
-
Genetic analysis of yeast Yip1p function reveals a requirement for Golgi-localized rab proteins and rab-Guanine nucleotide dissociation inhibitor.Genetics. 2004 Dec;168(4):1827-41. doi: 10.1534/genetics.104.032888. Genetics. 2004. PMID: 15611160 Free PMC article.
-
Yos1p is a novel subunit of the Yip1p-Yif1p complex and is required for transport between the endoplasmic reticulum and the Golgi complex.Mol Biol Cell. 2005 Apr;16(4):1673-83. doi: 10.1091/mbc.e04-10-0873. Epub 2005 Jan 19. Mol Biol Cell. 2005. PMID: 15659647 Free PMC article.
-
Multicopy suppressor analysis of thermosensitive YIP1 alleles implicates GOT1 in transport from the ER.J Cell Sci. 2009 May 15;122(Pt 10):1540-50. doi: 10.1242/jcs.042457. Epub 2009 Apr 21. J Cell Sci. 2009. PMID: 19383723 Free PMC article.
-
Vesicle transport: a close collaboration of Rabs and effectors.Curr Biol. 2004 Jan 6;14(1):R33-4. doi: 10.1016/j.cub.2003.12.021. Curr Biol. 2004. PMID: 14711434 Review.
-
A structural view of the COPII vesicle coat.Curr Opin Struct Biol. 2004 Apr;14(2):147-53. doi: 10.1016/j.sbi.2004.02.002. Curr Opin Struct Biol. 2004. PMID: 15093828 Review.
Cited by
-
Statistical analysis reveals co-expression patterns of many pairs of genes in yeast are jointly regulated by interacting loci.PLoS Genet. 2013 Mar;9(3):e1003414. doi: 10.1371/journal.pgen.1003414. Epub 2013 Mar 28. PLoS Genet. 2013. PMID: 23555313 Free PMC article.
-
Linking membrane trafficking and intestinal homeostasis.Tissue Barriers. 2013 Jan 1;1(1):e23119. doi: 10.4161/tisb.23119. Tissue Barriers. 2013. PMID: 24665373 Free PMC article.
-
A docked mutation phenocopies dumpy oblique alleles via altered vesicle trafficking.PeerJ. 2021 Oct 13;9:e12175. doi: 10.7717/peerj.12175. eCollection 2021. PeerJ. 2021. PMID: 34721959 Free PMC article.
-
RNA metabolism is the primary target of formamide in vivo.Sci Rep. 2017 Nov 21;7(1):15895. doi: 10.1038/s41598-017-16291-8. Sci Rep. 2017. PMID: 29162938 Free PMC article.
-
Large-scale profiling of Rab GTPase trafficking networks: the membrome.Mol Biol Cell. 2005 Aug;16(8):3847-64. doi: 10.1091/mbc.e05-01-0062. Epub 2005 Jun 8. Mol Biol Cell. 2005. PMID: 15944222 Free PMC article.
References
-
- Allan, B.A., B.D. Moyer, and W.E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding of COPII vesicles for fusion. Science. 289:444–448. - PubMed
-
- Ausubel, R.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1987. Current Protocols in Molecular Biology. Greene Publishing Associates and Wiley-InterScience, New York. 3.0.1–3.14.3.
-
- Baker, D., L. Hicke, M. Rexach, M. Schleyer, and R. Schekman. 1988. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 54:335–344. - PubMed
-
- Barlowe, C., L. Orci, T. Yeung, M. Hosobuchi, S. Hamamoto, N. Salama, M. Rexach, M. Ravazzola, M. Amherdt, and R. Schekman. 1994. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the ER. Cell. 77:895–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

