Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections
- PMID: 14557287
- PMCID: PMC207110
- DOI: 10.1128/CMR.16.4.569-596.2003
Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections
Abstract
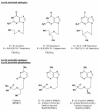
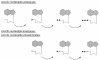
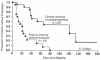
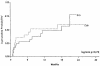
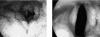
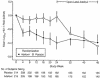
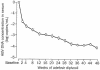
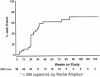
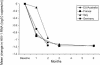
The acyclic nucleoside phosphonates HPMPC (cidofovir), PMEA (adefovir), and PMPA (tenofovir) have proved to be effective in vitro (cell culture systems) and in vivo (animal models and clinical studies) against a wide variety of DNA virus and retrovirus infections: cidofovir against herpesvirus (herpes simplex virus types 1 and 2 varicella-zoster virus, cytomegalovirus [CMV], Epstein-Barr virus, and human herpesviruses 6, 7, and 8), polyomavirus, papillomavirus, adenovirus, and poxvirus (variola virus, cowpox virus, vaccinia virus, molluscum contagiosum virus, and orf virus) infections; adefovir against herpesvirus, hepadnavirus (human hepatitis B virus), and retrovirus (human immunodeficiency virus types 1 [HIV-1] and 2 [HIV-2], simian immunodeficiency virus, and feline immunodeficiency virus) infections; and tenofovir against both hepadnavirus and retrovirus infections. Cidofovir (Vistide) has been officially approved for the treatment of CMV retinitis in AIDS patients, tenofovir disoproxil fumarate (Viread) has been approved for the treatment of HIV infections (i.e., AIDS), and adefovir dipivoxil (Hepsera) has been approved for the treatment of chronic hepatitis B. Nephrotoxicity is the dose-limiting side effect for cidofovir (Vistide) when used intravenously (5 mg/kg); no toxic side effects have been described for adefovir dipivoxil and tenofovir disoproxil fumarate, at the approved doses (Hepsera at 10 mg orally daily and Viread at 300 mg orally daily).
Figures

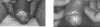
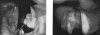








References
-
- Abdulkarim, B., S. Sabri, E. Deutsch, H. Chagraoui, L. Maggiorella, J. Thierry, F. Eschwege, W. Vainchenker, S. Chouaïb, and J. Bourhis. 2002. Antiviral agent cidofovir restores p53 function and enhances the radiosensitivity in HPV-associated cancers. Oncogene 21:2334-2346. - PubMed
-
- Aldern, K. A., S. L. Ciesla, K. L. Winegarden, and K. Y. Hostetler. 2003. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-14C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63:678-681. - PubMed
-
- Andrei, G., R. Snoeck, D. Schols, and E. De Clercq. 2001. Induction of apoptosis by cidofovir in human papillomavirus (HPV)-positive cells. Oncol. Res. 12:397-408. - PubMed
-
- Andrei, G., R. Snoeck, E. De Clercq, R. Esnouf, P. Fiten, and G. Opdenakker. 2000. Resistance of herpes simplex virus type 1 against different phosphonylmethoxyalkyl derivatives of purines and pyrimidines due to specific mutations in the viral DNA polymerase gene. J. Gen. Virol. 81:639-648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

