Antigens and alternatives for control of Anaplasma marginale infection in cattle
- PMID: 14557295
- PMCID: PMC207124
- DOI: 10.1128/CMR.16.4.698-712.2003
Antigens and alternatives for control of Anaplasma marginale infection in cattle
Abstract
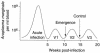
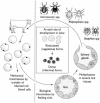
Anaplasmosis, a tick-borne cattle disease caused by the rickettsia Anaplasma marginale, is endemic in tropical and subtropical areas of the world. The disease causes considerable economic loss to both the dairy and beef industries worldwide. Analyses of 16S rRNA, groESL, and surface proteins have resulted in the recent reclassification of the order Rickettsiales. The genus Anaplasma, of which A. marginale is the type species, now also includes A. bovis, A. platys, and A. phagocytophilum, which were previously known as Ehrlichia bovis, E. platys, and the E. phagocytophila group (which causes human granulocytic ehrlichiosis), respectively. Live and killed vaccines have been used for control of anaplasmosis, and both types of vaccines have advantages and disadvantages. These vaccines have been effective in preventing clinical anaplasmosis in cattle but have not blocked A. marginale infection. Thus, persistently infected cattle serve as a reservoir of infective blood for both mechanical transmission and infection of ticks. Advances in biochemical, immunologic, and molecular technologies during the last decade have been applied to research of A. marginale and related organisms. The recent development of a cell culture system for A. marginale provides a potential source of antigen for the development of improved killed and live vaccines, and the availability of cell culture-derived antigen would eliminate the use of cattle in vaccine production. Increased knowledge of A. marginale antigen repertoires and an improved understanding of bovine cellular and humoral immune responses to A. marginale, combined with the new technologies, should contribute to the development of more effective vaccines for control and prevention of anaplasmosis.
Figures



References
-
- Abdala, A. A., E. Pipano, D. H. Aguirre, A. B. Gaido, M. A. Zurbriggen, A. J. Mangold, and A. A. Guglielmone. 1990. Frozen and fresh Anaplasma centrale vaccines in the protection of cattle against Anaplasma marginale infection. Rev. Elev. Med. Vet. Pays Trop. 43:155-158. - PubMed
-
- Aguirre, D. H., A. C. Bermúdez, A. J. Mangold, and A. A. Guglielmone. 1988. Infección natural con Anaplasma marginale en bovines de raza Hereford, Criolla and Nelore en Tucumán, Argentina. Rev. Latinoam. Microbiol. 30:37-42. - PubMed
-
- Aguirre, D. H., A. B. Gaido, A. A. Abdala, L. G. de Ríos, A. J. Mangold, and A. A. Guglielmone. 1988. Evaluación de la protección conferida contra Anaplasma marginale por una vacuna de A. marginale muerto, una vacuna de Anaplasma centrale vivo y una combinación de ambas en bovinos Holando Argentino. Rev. Med. Vet. (Buenos Aires) 69:13-19.
-
- Aguirre, D. H., A. J. Mangold, L. G. de Ríos, and A. A. Guglielmone. 1991. Respuesta clínica y evolución del peso corporal (Bos taurus) vacunadas simultáneamente contra babesiosis y anaplasmosis con inmunógenos vivos. Med. Vet. (Barcelona) 8:95-101.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

